Starch
Starch is the main carbohydrate in cereal grains and it can be considered to be the most important malt component. To make beer, the starch in the grain is first broken apart by milling. The exposed starch then undergoes changes during mashing including gelatinization, liquefaction, and finally saccharification. This degradation process yields fermentable and non-fermentable sugars. The fermentable sugars ultimately become alcohol and carbon dioxide gas. If the starch degradation is incomplete for some reason, intact starch molecules can cause a hazy beer. Starch and its polysaccharide degradation products are sometimes called α-glucans, because of the alpha linkages they contain (β-glucans have beta linkages between the glucose units).
Starch structure and organization
-
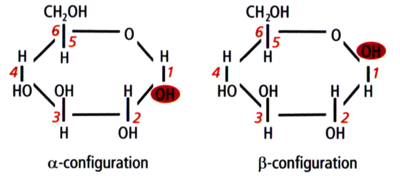
Glucose in alpha and beta configurations
-
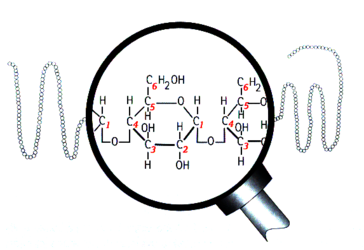
Structure of amylose
-
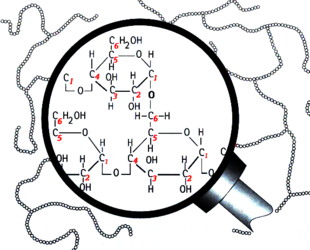
Branching in the structure of amylopectin
Structurally, starch is a polymer of glucose that can be classified into two categories: amylose and amylopectin. Amylose makes up 20–30% of the starch in barley and it consists of long unbranched or slightly-branched chains.[1][2][3] The rest is amylopectin, a much larger molecule consisting of branching chains.[4][5][6][7][8] Learning the types of bonds within the starch molecules is important to understand enzyme activity in the mash and ultimately the makeup of wort and beer. The most common bond between the glucose units in starch is the α-1,4 glucosidic bond, which forms a straight chain. Branch points are formed by α-1,6 glucosidic bonds. FYI, this bond naming convention comes from the numbering of the carbon atoms in the glucose molecule. For example, 1,4 bonds are a connection between the 1st and 4th carbons of adjacent glucose units (see the above illustrations). The branch points are responsible for the unfermentable sugars (dextrins) in wort because the active enzymes in the mash generally cannot break down the starch molecules near a branch. Therefore, the degree of starch branching is a key variable that may impact beer quality, but unfortunately it is not measured by maltsters or brewers.[8]
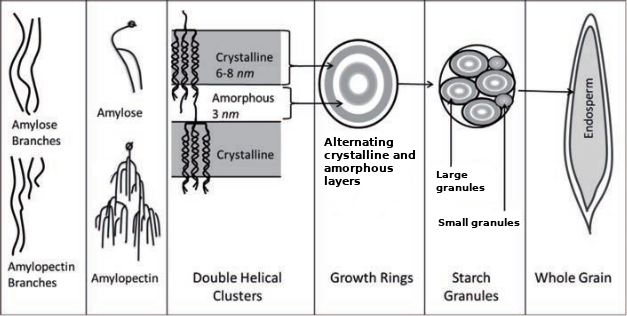
In cereal grain, the starch molecules form layers of crystalline and amorphous structure in order to create granules, which make up the endosperm.[9] Starch granules in barley can be divided into two fractions, with average particle sizes of around 20 µm for large granules and 2 µm for small granules.[10][3] These types of barley starch granules are characterized by different amylose:amylopectin ratios, as well as different structures and properties—starch gelatinization temperature in particular.[11][12] The "large" starch granules contain 70–95% of the starch mass.[13][14] This information is useful to help understand the impact that granule sizes have on gelatinization, namely that the "small" granules have a significantly higher gelatinization temperature, which may ultimately require a step mash to extract.[15][2][16][17]
Science
The ends of the starch chains differ in their structure due to the asymmetrical nature of the glucose molecule. Carbon 1 of the glucose unit is the "reducing end" (RE) and carbon 4 is the "non-reducing end" (NRE). Amylose has one RE and one NRE, while amylopectin has one RE and many NREs.[18] The ends differ in their chemistry and shape, which affects how the enzymes interact with the ends of the chains.
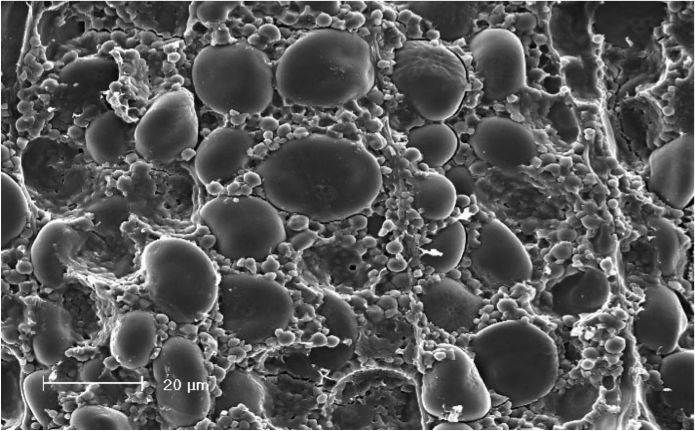
Gelatinization
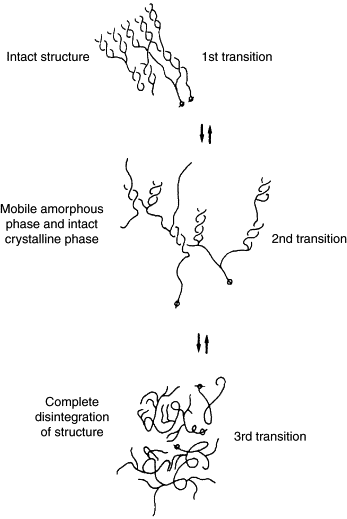
Gelatinization (also called "pasting"), which typically occurs during mashing, is the hydration of starch molecules. When exposed to hot water, starch granules swell and burst, losing their organized structure (think popcorn).[19][20][21][2] This physical process makes the starch much more accessible to enzymes.[22][23][3] If the starch is not fully gelatinized, it can potentially result in a lower yield, lower attenuation, filtration difficulties, possible scorching, and starch haze.[19][22] Therefore, the gelatinization temperature is important to consider when choosing a mashing schedule.[24] In the mash, gelatinization is followed by liquefaction and saccharification, an enzymatic process.
The gelatinization temperature of most cereals is between 149–176°F (65–80°C). However, it drops noticeably in the presence of starch-degrading enzymes (i.e. in malted grain).[19] For example, barley malt starch normally begins to gelatinize at 138–142°F (59–61°C), although it is variable.[19][25] It's important to understand that not all of the starch in a batch gelatinizes at the same temperature.[3] In particular, the "small" starch granules gelatinize at a significantly higher temperature than the majority of starch from the "large" granules, potentially as high as 186°F (80°C) in the case of barley malt.[20][23][26] Also be aware that different batches of grain will have slightly different gelatinization temperatures due to the effects of variable growth conditions and climatic influences.[24][27][28] Furthermore, the gelatinization temp can be influenced by milling—more intensive crushing lowers the gelatinization temperature.[23][29][30]
It can be problematic if too much starch in a batch of malt gelatinizes above 149°F (65°C).[3] β-amylase and limit dextrinase are quickly inactivated above this threshold, which reduces the amount of starch degradation and therefore reduces the fermentability of the wort and/or decreases the yield.[31][32] Even with a proper step mash, this may result in a lower final attenuation as well as negative effects on flavor and mouthfeel.[24][33]
For "micronized", "torrefied", and "flaked" grains, the starch is already gelatinized (as part of the production process) and these grain products can be added directly to a normal malt mash in reasonable quantities (see Adjuncts).[34] With the exception of wheat, oats, and rye, raw unmalted cereals typically require a separate high-temperature gelatinization step to be used in a mash because the gelatinization temperature is too high to achieve during a normal mashing process (see Cereal mash).[21][3]
| Grain | Typical gelatinization temperature |
|---|---|
| Barley malt | 140–149°F (60–65°C)[20][21][22][27][35][36][3] |
| Wheat | 126–147°F (52–64°C)[35][3] |
| Oats | 126–147°F (52–64°C)[3] |
| Rye | 120–142°F (49–61°C)[3] |
| Maize | 143–176°F (62–80°C)[35][3] |
| Sorghum | 156–185°F (69–85°C)[35][3] |
| Rice | 142–180°F (61–82°C)[35][3] |
| Potato | 133–156°F (56–69°C)[35] |
See also
References
- ↑ Buléon A, Colonna P, Planchot V, Ball S. Starch granules: structure and biosynthesis. Int J Biol Macromol. 1998;23(2):85–112.
- ↑ a b c Bamforth CW, Fox GP. Critical aspects of starch in brewing. BrewingScience. 2020;73(9/10):126–139.
- ↑ a b c d e f g h i j k l m Evans E. Mashing. American Society of Brewing Chemists and Master Brewers Association of the Americas; 2021.
- ↑ Kunze W. Raw materials. In: Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:39–107.
- ↑ Meussdoerffer F, Zarnkow M. Starchy raw materials. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ Miedl-Appelbee M. Brewhouse technology. In: Stewart GG, Russell I, Anstruther A, eds. Handbook of Brewing. 3rd ed. CRC Press; 2017.
- ↑ Holbrook CJ. Brewhouse operations. In: Smart C, ed. The Craft Brewing Handbook. Woodhead Publishing; 2019.
- ↑ a b Fox GP. Starch in brewing applications. In: Sjöö M, Nilsson L, eds. Starch in Food. 2nd ed. Woodhead Publishing; 2017:633–659.
- ↑ Ratnayake WS, Jackson DS. Starch Gelatinization. In: Advances in Food and Nutrition Research. Academic Press; 2008;55:221–268.
- ↑ Chmelík J, Krumlová A, Budinská M, et al. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field-flow fractionation. J Inst Brew. 2001;107(1).
- ↑ Szwajgier D. "Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts." J Inst Brew. 2011;117(4):569–577.
- ↑ Yu W, Zhai H, Xia G, et al. Starch fine molecular structures as a significant controller of the malting, mashing, and fermentation performance during beer production. Trends Food Sci Technol. 2020;105:296–307.
- ↑ Krottenthaler M, Back W, Zarnkow M. Wort production. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ Langenaeken NA, De Schepper CF, De Schutter DP, Courtin CM. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019;295:138–146.
- ↑ Keßler M, Kreisz S, Zarnkow M, Back, W. Do brewers need a starch modification index? Brauwelt International. 2008;1:52–55.
- ↑ Slack PT, Baxter ED, Wainwright T. Inhibition by hordein of starch degradation. J Inst Brew. 1979;85(2):112–114.
- ↑ Cozzolino D, Degner S. An overview on the role of lipids and fatty acids in barley grain and their products during beer brewing. Food Res Int. 2016;81:114–121.
- ↑ Lewis MJ, Young TW. Brewing. Springer; 2001:234.
- ↑ a b c d Kunze W. Wort Production. In: Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:220–221.
- ↑ a b c Krottenthaler M, Back W, Zarnkow M. Wort production. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ a b c Fix G. Principles of Brewing Science. 2nd ed. Brewers Publications; 1999.
- ↑ a b c Slack PT, Wainwright T. Amylolysis of large starch granules from barleys in relation to their gelatinisation temperatures. J Inst Brew. 1980;86:74–77.
- ↑ a b c Mousia Z, Balkin RC, Pandiella SS, Webb C. The effect of milling parameters on starch hydrolysis of milled malt in the brewing process. Process Biochem. 2004;39(12):2213–2219.
- ↑ a b c Sacher B, Becker T, Narziss L. Some reflections on mashing – Part 2. Brauwelt International. 2016;6:392-397.
- ↑ MacGregor AW, Bazin SL, Macri LJ, Babb JC. Modelling the contribution of alpha-amylase, beta-amylase and limit dextrinase to starch degradation during mashing. J Cereal Sci. 1999;29(2):161–169.
- ↑ Langenaeken NA, De Schepper CF, De Schutter DP, Courtin CM. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019;295:138–146.
- ↑ a b Narziss L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
- ↑ Pahl R, Meyer B, Biurrun R. Wort and Wort Quality Parameters. In: Bamforth CW, ed. Brewing Materials and Processes: A Practical Approach to Beer Excellence. Academic Press; 2016.
- ↑ Morrison WR, Tester RF, Gidley MJ. Properties of damaged starch granules. II. Crystallinity, molecular order and gelatinisation of ball-milled starches. J Cereal Sci. 1994;19(3):209–217.
- ↑ Warpala IWS, Pandiella SS. Grist fractionation and starch modification during the milling of malt. Food and Bioproducts Processing. 2000;78(2):85–89.
- ↑ Evans DE, Fox GP. Comparison of diastatic power enzyme release and persistence during modified Institute of Brewing 65°C and Congress programmed mashes. J Am Soc Brew Chem. 2017;75(4):302–311.
- ↑ Evans DE, Li C, Eglinton JK. The properties and genetics of barley malt starch degrading enzymes. In: Zhang G, Li C, eds. Genetics and Improvement of Barley Malt Quality. Springer; 2010:143–189.
- ↑ Evans DE, Collins H, Eglinton J, Wihelmson A. Assessing the impact of the level of diastatic power enzymes and their thermostability on the hydrolysis of starch during wort production to predict malt fermentability. J Am Soc Brew Chem. 2005;63(4):185–198.
- ↑ Howe S. Raw materials. In: Smart C, ed. The Craft Brewing Handbook. Woodhead Publishing; 2019.
- ↑ a b c d e f Meussdoerffer F, Zarnkow M. Starchy raw materials. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.