Sulfite


Please check back later for additional changes

Sulfites (including sulfur dioxide [SO2]) are additives used in both wine and beer production for their antioxidant and anti-microbial effects. These actions make sulfites useful for a variety of tasks including preventing oxidation, inhibiting microbes, quickly removing chlorine compounds from tap water, and even sanitizing brewing equipment. Sulfites are also a natural product of yeast fermentation, and therefore they are present in every fermented beverage. Forget everything you thought you knew about sulfites; misinformation is rampant in common online sources and even some books.
Sulfites are NOT directly responsible for the sulfurous/rotten egg/burnt match aroma, as is commonly mistaken — hydrogen sulfide and/or ethyl mercaptan are the sources of that off-flavor.[1][2][3] Sulfites should not be confused with sulfate or sulfide.
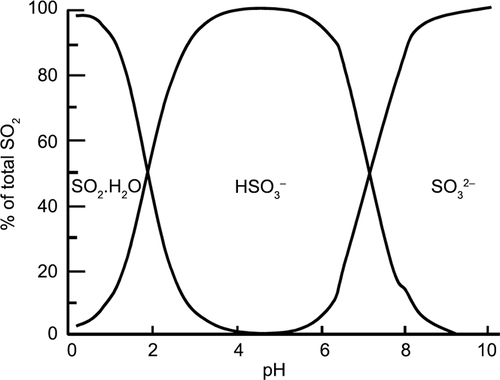
Sulfur dioxide (SO2) is a gas that is 85 g L−1 soluble in water at 25 °C and has a boiling point of −10 °C.170 In solution, it undergoes equilibrium reactions with SO2.nH2O, the bisulfite ion (HSO3 −), and the sulfite ion (SO3 −). At beer pH, which is generally 3.8−4.4, the predominant form is the bisulfite ion.158,171 Because all of these species can be converted to, measured as, and reported in terms of SO2, they are often generalized under “SO2” or “sulfites”.[4]
The use of radical scavengers could improve beer flavor stability.[5] Sulfite has been identified as an essential antioxidant in beer, which has been ascribed to its ability to remove H2O2.[6]
Sources of Sulfite[edit]
the major source of sulfite in beer is the reduction of sulfate in water and grist by the yeast metabolism (endogenous SO2). The SO2 content is also increased by the addition of sulfiting agents (exogenous SO2) such as SO2 (E220), Na2SO3 (E221), NaHSO3 (E222), Na2S2O5 (E223), K2S2O5 (E224), CaSO3 (E226), Ca(HSO3)2, (E227), and KHSO3 (E228) before beer packaging.115,158,171−173 According to Johannesen et al.,175 no difference could be noticed between the (E)-2-nonenal concentrations of forced-aged beer with sulfite derived from endogenous or exogenous origin.175[4]
Products[edit]
Sulfite is available in powdered form as sodium metabisulfite and potassium metabisulfite, and also in tablet form with the brand name Campden. These products are not entirely interchangeable, so it's important to note their differences when selecting a product. Neither potassium nor sodium affect the action of the sulfite, but they can have other effects.
- Sodium metabisulfite (Na2S2O5 also known as Na-meta or SMS) powder is 67% SO2 by weight. The sodium can affect flavor.
- Potassium metabisulfite (K2S2O5 also known as K-meta or KMS) powder is 58% SO2 by weight. The potassium is flavor neutral, so it is generally preferred in wine making. Potassium can also help some wines by precipitating with tartrate salts (see adjusting acidity in wine). However it may be less favorable in beer brewing because high levels of potassium are known to inhibit enzymes during the mash.
- Campden tablets come in different strengths and can be either sodium or potassium metabisulfite. If you use tablets, be aware of what form and strength they are.
- Sulfur discs or sticks can be burned to treat oak barrels. (The use of these products is not covered in this article.)
[Insert product links]
- Caution
Sulfite products (especially powder) give off SO2 gas that causes a choking sensation. Try to avoid inhaling the fumes.[7] If you have asthma, be especially cautious and use in a well-ventilated area.
- Proper Storage
Because sulfite reacts with oxygen, it needs to be stored in an air-tight container with as little air and moisture exposure as possible, otherwise it loses its potency.[8] The shelf life is limited to 6-12 months even with careful storage.[9][10][11][12][13] The powder becoming clumpy is a sign of exposure to moisture, and therefore degradation. Odor detection/evaluation is NOT a good indicator of product quality because it does not tell you how much degradation has occurred.
Natural Sulfite[edit]
Sulfite comprises an intermediate product of cysteine and methionine biosynthesis, and its excretion by yeast proceeds in four stages22,171,175,188,189 (Figure 20). In stage 1, methionine and threonine present in wort inhibit and repress certain enzymes, preventing sulfite excretion. During the second stage, the pathway is switched on, but sulfite excretion remains low due to a high demand for sulfur-containing amino acids. In stage 3, yeast growth ceases, which lowers this amino acid demand. However, extract, and thus energy, is still available, which favors sulfite production. Sulfite excretion commences due to an oversupply in the metabolism. The alcohol level at this moment is about 1.5% w/w.75,190 In the fourth stage, the extract is depleted, sulfate reduction stops, and sulfite excretion stops accordingly.189 The extent of sulfite excretion depends on the yeast strain used; lager strains often produce more SO2 than ale strains, for example.191 It has been found that beer produced with a yeast strain with augmented sulfite secretion shows better flavor stability.192 Furthermore, higher sulfate supply to the yeast, higher original wort gravity, higher wort clarity, higher fermentation temperature, lower pitching rate, and lower wort oxygenation all result in higher SO2 contents.158,171,189,190 In general, sulfite secretion is inversely proportional to yeast growth, independent of the applied parameters.189[4]
Sulfite is produced naturally by yeast during fermentation, and may be present at the end of fermentation in some amount, usually less than 30ppm although some strains can produce vastly higher amounts.[14][15][6] Yeast produce sulfite by reducing sulfate, although the concentration of sulfate may have only a minor effect on the amount of sulfite produced, depending on the yeast strain. Yeast also produce compounds during fermentation that bind to sulfite, decreasing the proportion of free SO2.
Sulfite is produced by the yeast during fermentation and is an important antioxidant in beer.[16][6] This natural sulfite production is promoted by must/wort clarity and low aeration.[17]
- Andersen, M. L., Outtrup, H., and Skibsted, L. H. Potential antioxidants in beer assessed by ESR spin trapping. J. Agric. Food Chem. 48:3106-3111, 2000.
- Uchida, M., and Ono, M. Improvement for oxidative flavor stability of beer—Role of OH-radical in beer oxidation. J. Am. Soc. Brew. Chem. 54:198-204, 1996.
The formation of extracellular SO2 does not occur until nutrient repression and biomass formation has ceased, toward the middle of fermentation (60). It is important to note that SO2 formation is impacted by wort composition and yeast strain selection; ale strains are effectively nonproducers.[17]
Sulphite is considered to prevent the oxidative staling. of beer17,31,34. Sulphite is produced by yeast during fermentation4,33.[18]
Dufour (13) found sulfite levels to be dependent on total fermenting biomass that decreased with the rate of fermentation by the yeast.[19]
studies have shown that the carbohydrate addition prior fermentation leads to an increase of the osmotic pressure resulting in a stronger SO2-formation during fermentation. This fact should have a positive effect on the oxidative beer stability because sulphur dioxide can act as an significant antioxidant in the beer matrices by scavenging ROS and masking stale flavour by binding staling aldehydes as sulphite carbonyl complexes [4, 9, 15, 28, 45–48, 50, 55, 61–63].[20]
In beer containing sulfites, free radicals are generated after a definite time period, called the "lagtime".[21]
Brighter worts allow increased levels of SO 2 production by yeast.[22]
sulfite is an important antioxidant for improving beer flavor stability, particularly in combination with the phenolic compounds.[23] There is an interaction between SO2 and some polyphenols that retard the loss of SO2 and consequently, a rapid beer ageing. This interaction seems to be reversible since the free SO2 concentration is similar in fresh and aged beer; however there is a large difference in total SO2 concentration. The polyphenols have an indirect and positive role in beer ageing because they avoid a rapid SO2 lost and by its metal chelating capacity.
The aldehyde-sulfite adducts that may be formed from saturated aldehydes in beer are most likely not active antioxidants but may act simply as reservoirs of sulfite, whereas R,â-unsaturated aldehydes are able to bind sulfite irreversibly (Dufour et al., 1999; Nyborg et al., 1999).[24]
4ppm sulfite is sufficient to act as an antioxidant, delaying oxidation.[24]
Sulfite is a clearly a unique antioxidant in beer. It is formed naturally by the yeast during the fermentation, and according to the present study, it is the most efficient antioxidant that is naturally present in beer (Ilett, 1995; Kaneda et al., 1996). Beer lacking sulfite was found to have no lag phase for formation of radicals and accordingly no defense against the oxidative radical chain reactions. Sulfite either added as such or bound by carbonyl compounds as 1-hydroxysulfonates resulted in a lag phase. The lability of such carbonyl adducts ensure that the antioxidative effect of sulfite is not reduced.[24]
Sulfite Usage in Wine[edit]
Because of its utility, sulfite is an extremely common additive in commercial wines as well as homemade wine. It can be added at different points in the process to prevent of oxidation and/or control wild microbes. Sulfite usage does not damage the flavor of a wine unless excessive quantities are used that put it above flavor threshold. It is practically a necessity for a wine that will be aged because oxidation will significantly damage wine quality over time.
Although sulfite does have anti-microbial action, it does not kill most wild microbes when it is used in reasonable concentrations. It works largely by inhibition, which means that proper levels need to be maintained in order for it to be effective. The anti-microbial action is dependent not just on the sulfite level, but also on the pH of the wine or must.
Sulfite in Must (Pre-Fermentation)[edit]
Sulfite dosage rate is NOT one-size-fits-all. The amount should be tailored to the pH of the must. Using sulfite without measuring the pH may yield highly unpredictable results ranging from spoiled must to a fermentation that won't start.
If You Crush Your Own Fruit[edit]
Many (but not all) winemakers add sulfite at crush to prevent oxidation. This also serves to increase oxygen available to the yeast during fermentation.
Since the sulfite needs to be added as soon as possible (e.g. as the fruit comes out of the crusher) to prevent oxidation, exact pH testing is not always utilized and therefore more general rates are used. The sulfite should be dissolved in water before adding to the fruit to ensure it is evenly distributed. Oxidation literally occurs within minutes, so there are diminishing returns (for preventing oxidation) when adding sulfite any later in the pre-fermentation process.
| Healthy fruit, Low pH | Healthy fruit, High pH | Fruit with some rot | |
|---|---|---|---|
| White | 25-50 ppm | 60-80 ppm | 80-100 ppm |
| Red | 50 ppm | 50-80 ppm | 80-100 ppm |
*Increase these amounts for unclarified musts, but do not exceed 100ppm total SO2.
If pH testing is utilized (e.g. on a smaller sample before the main crush), a tailored dose can be used:
Instructions
- Measure the pH of the must to within 0.1. Using a pH meter calibrated before use is best (pH strips can be inaccurate).
- If the pH is above 3.8, consider adjusting it down to 3.8 with the acid of your choice if microbial control is desired. See adjusting acidity in wine.
- Determine your target molecular SO2 level:
- Calculate the amount of free SO2 needed. To determine the amount of sulfite that needs to be added, use the sulfite calculator at FermCalc. Target no less than 25ppm free SO2.
- Measure the sulfite with a scale if using a powdered form or count and crush the appropriate number of tablets.
- Gently dissolve the sulfite in water before adding it to the must. Briefly give it a gentle stir.
- The must should sit with the sulfite for approximately 24 hours.[26][25] It should be left open to air during this time to allow some of the sulfite to gas off; cover with a towel or other method to prevent insects from getting in the must.
- Prior to pitching yeast, the must needs to be thoroughly aerated in order to help neutralize some of the excess sulfite so that it doesn't affect the pitched yeast, and to provide the yeast with oxygen.
If You Obtain Unpasteurized Juice or Make Mead with Raw Honey[edit]
Sulfite usage before fermentation may be used to inhibit or kill a portion the wild microbes naturally present on and inside the fruit or other ingredients like raw honey. The wild microbes can be beneficial for wine in various ways,[27] so the winemaker should consider whether inhibiting them is needed. Attempting to prevent oxidation may or may not be worthwhile if sulfite wasn't added during the crush.
Follow the same instructions as described above.
If You Use Pasteurized Ingredients[edit]
If all the ingredients in the must (fruit, juice, honey, etc) are pasteurized, then there are no wild microbes and oxidation enzymes have been destroyed. Therefore, in this case sulfite before fermentation is certainly not needed and should not be used because it serves no purpose.
Sulfite After Fermentation[edit]
Sulfite can be added after fermentation to protect the wine during aging, both by preventing oxidation as well as inhibiting microbial activity (mainly bacteria and Brettanomyces). Be aware that adding sulfite during an active fermentation will likely not stop the fermentation and may have detrimental effects.[14]
If Brettanomyces flavor development is desired or if the wine will be naturally carbonated via bottle conditioning, then sulfite probably should not be used because it will inhibit the respective yeast and may lead to off-flavors. Fortunately, both Brettanomyces and the process of bottle conditioning are naturally helpful for preventing or reducing oxidation.
Sulfite should be added after the primary fermentation and malolactic fermentation are completed. Depending on how long the wine is aged and how it is handled, sulfite levels may need to be increased periodically (every 1 to 3 months).[28] This largely depends on the individual system. Measuring free SO2 is very helpful for making the determination as to when additional sulfite is needed and how much.
The level of free SO2 should generally be maintained in the 20-80ppm range during aging in order to prevent oxidation.[14][28] Molecular SO2 should be maintained above 0.6ppm to inhibit unwanted microbial activity. White wines and sweet wines (or any wine with low tannins) generally need higher levels of molecular SO2 to inhibit microbes. Be aware that after the wine is packaged, the sulfite level will continue to decline.
[Need info: Accounting for binding] Most experts suggest to expect between 30-50% binding when total SO2 is under 100ppm, with the bound fraction diminishing as the total SO2 increases.[14] Divide the amount of sulfite you add by the fraction of sulfite that you expect to be free (e.g. if you want to assume 30% binding, divide the amount of sulfite you are adding by 0.7). (See sulfite binding below.)
The ultimate goal is to have the molecular SO2 level around 0.5-0.8ppm at the time the wine is consumed. This level assures that the wine is continually protected from wild microbes and that the SO2 level is well below taste threshold. Achieving this level may take some trial and error because it can vary from wine to wine and system to system. Levels of molecular SO2 above 1.0-2.0 may cause faults such as a sulfurous aroma or even a noxious choking sensation when inhaling the aroma. Free SO2 generally does not cause off flavors/aromas. FermCalc is extremely helpful for calculating sulfite adjustments. If the wine pH is above 3.8, it should be adjusted downward with acid in order to increase the relative proportion of molecular SO2, which lowers the amount of free/total SO2 that is needed.
Instructions (for clean and still wine):
- Wait for fermentation to complete in primary vessel.
- Measure the pH and the sulfite level.
- Use FermCalc to calculate the amount of sulfite needed to reach the target free SO2 (20-80ppm). Molecular SO2 should be around 0.6-1.0ppm.
- Increase the amount to account for binding.
- Measure, dissolve, and add the sulfite to the wine. If racking, add it to the secondary vessel while transferring the wine.
- Repeat steps 2-5 at least every 1-3 months, particularly whenever transferring the wine and when bottling.
Sulfite should always be used when stabilizing a wine by adding sorbic acid. This helps prevent off flavors from developing.
[Insert instructions for people not measuring sulfite levels... or pH?]
Sulfite Usage in Beer[edit]
Sulfites play a significant role in masking stale flavor, and in protecting beer from oxidation and slightly from microbial spoilage [7, 8, 12, 14, 16, 27, 30, 38, 47, 52, 54, 55]. There are two factors that promote the positive effect of SO2 on the flavor stability of beer. SO2 or sulfite (HSO3-) is a reactive antioxidant, which reduces oxygen and therefore causes a better endogenous antioxidative potential [2, 18, 30, 34, 35, 46, 54, 55, 56]. Furthermore SO2 is building reversible complexes with carbonyls which cause a masking effect since these carbonyls are mainly ageing flavour related compounds [1, 2, 5, 11, 14, 23, 26, 30, 39, 40, 52].[29]
A multitude of studies have described specific fermentation parameters that affect the sulphite formation by yeast in wine and beer production. Thereby, the wort aeration plays a key role during fermentation. But also the effect of pitching rate, temperature and pressure on the level of sulphur dioxide formation has been investigated by different research groups [6, 15, 34, 36]. The results of these investigations are in line with Kaneda et al. [23, 26, 43], who showed that the wort fermentation conditions are influencing the sulfite level and therefore also the flavor stability of the finished beer by inhibiting radical reactions and oxidative processes. Furthermore the sulfite is able to mask staling fl avour by formation of sulphite complexes with ageing flavor related carbonyls [1, 2, 13, 34]. In this context Narziss et al. [41] and Foster et al. [18] recommended that sulphur dioxide content in packaged beer of 8–9/6-7 mg/L generated by yeast is most appropriate for the flavor stability of beer.[29]
Higher levels of CO2 can increase SO2 production.[29]
Sulfite is typically used in low oxygen brewing for its ability to actively scavenge oxygen and prevent oxidation.[30][31]
Sulfite is not a very effective reducing agent toward disulfides in beer.[16] This may be explained by the low pH in beer because the disulfide reducing capacity of sulfite has been found to be optimal at pH 7.0 and, therefore, effectively reduced with decreasing pH.
Outside of the context of low oxygen brewing, sulfite is rarely used in beer production, with the exception of chlorine removal from water.
One study showed that the SO2 level in 40 commercial lagers was not correlated with antioxidant activity.[32] Levels ranged from zero to 37 mg/L.
Sulfite is an important constituent of beer and is produced by yeast during fermentation and survives into the finished beer. Thus, the differences of brewer’s yeast and brewing process would lead to significant differences in SO2 concentrations of the final beers. Previous studies showed that wort fermentation conditions play an important role in the flavour stability of beer by controlling the sulfite level.24 Sulfite produced during fermentation influences the flavour staling of finished beer and a concentration of 8–9 mg L−1 of sulfite in packaged beer was considered as a good target level for flavour stability of beer by some researchers.25[32]
The addition of methionine reduces the production of SO2 (Van Haecht & Dufour, 1995); on the contrary, the addition of aspartic acid, threonine, cysteine and serine can increase the production of SO2 (Korch, Mountain, Gyllang, Winge, & Brehmer, 1991; Van Haecht & Dufour, 1995; Yoshida et al., 2008).[33]
Sulfur dioxide (SO2 ) is a powerful antioxidant, though the levels in beer permitted by regulation (10 mg/l in the USA) are generally insufficient to confer flavor stability; it forms addition compounds with aldehydes and so in beer is mostly bound SO2 . SO2 arises in beer by yeast action and is a variable depending on yeast strain, or arises by addition of KMS in the kettle or post- fermentation. It is an effective flavor preservative at levels somewhat below its flavor threshold of about 25 mg/l.[34]
It is generally accepted that sulfites protect beer from staling in two different ways.23,43,158,171,176−178 First, they can act as antioxidants, improving beer flavor stability by inhibiting oxidative chain reactions through radical scavenging of both ROS and other radicals. Sulfite seems to interact with peroxides in a two-electron nonradical producing reaction, preventing the formation of staling aldehydes and many other undesired products.21,179 Second, they have a role as carbonyl-binding agents through the formation of aldehyde−bisulfite adducts, the so-called hydroxysulfonates (Figure 18). As an illustration, the addition of sulfite to fresh beers strongly delayed the appearance of cardboard flavor during beer aging, and the level of free flavor-active (E)-2-nonenal lowered upon addition. Free SO2 disappears from beer over time, with a very low, but nonzero rate, at 0 °C, and faster with increasing temperature, following first-order kinetics. These rates are barely affected by the initial SO2 content.183 Free SO2 is most likely lost as an antioxidant pool, but likely also as a pool for binding de novo formed aldehydes or aldehydes released from, for example, imine adducts,38,176,183 as well as reversible or irreversible interaction with a whole range of other components such as reducing sugars, Maillard intermediates (thus inhibiting the Maillard cascade), cysteine residues, thiamins, quinones, and polyphenols. As acetaldehyde represents >95% of all aldehydes in beer, the majority of carbonyl-reacted SO2 will be associated with this compound. It has been suggested that most of (E)-2-nonenal is bound as a sulfite adduct as long as the total amount of SO2 in aging beer exceeds 2 mg/L. For the total carbonyl content, a maximum of 40% appears to be bound when 5−10 mg L−1 sulfite is added, which has been mentioned by Bushnell et al.180 as the optimal sulfite concentration in beer. Kaneda et al.177 found a similar optimal sulfite content in packaged beer, being 8−9 mg L−1 . From the above, it is clear that the precise role of SO2 in beer flavor stability is complex and that additional research is required. For instance, it has been mentioned that acetaldehyde−bisulfite adducts still show antioxidant activity in aging beer, protecting other compounds from oxidation,177,188 and it has even been proposed by Kaneda et al.188 that this activity may be more important than the actual carbonyl scavenging ability of sulfite.[4]
- Martinez-Periñan E, Hernández-Artiga MP, Palacios-Santander JM, El Kaoutita M, Naranjo-Rodriguez I and Bellido-Milla D, Estimation of beer stability by sulphur dioxide and polyphenol determination. Evaluation of a Laccase–Sonogel–Carbon biosensor. Food Chem 127:234–239 (2011).
- Ilett DR and Simpson WJ, Loss of sulphur dioxide during storage of bottled and canned beers. Food Res Int 28:393–396 (1995).
- Kaneda H, Takashio M, Osawa T, Kawakishi S and Tamaki T, Behavior of sulfites during fermentation and storage of beer. J Am Soc Brew Chem 54:115–120 (1996).
- Narziss L, Miedaner H, Graf H, Eichhorn P and Lustig S, Technological approach to improve flavour stability. MBAA Tech Q 30:48–53 (1993).
- Guido. (2005). How do sulfites help to control beer ageing. Cerevisia, 30, 132–183.
- Zhou. (2010). Study on the antioxidative effect of so2 and so2 in beer. Jinan, China: Shandong Agricultural University. MA. Sc.
- https://www.osti.gov/biblio/4156105 https://www.osti.gov/servlets/purl/4156105
Oxygen Scavenging in Beer[edit]
Sulfite increases the amount of protein free thiols available in wort and beer, which also serve as antioxidants.[16][35]
Low Oxygen Brewing[edit]
In low oxygen brewing, sulfite is added immediately before dough-in to help prevent oxidation during wort production (along with other oxygen-scavenging agents and process modifications). When beginning the transition to low oxygen brewing, the suggested starting amount of sulfite in the mash is 20-30ppm of sodium metabisulfite, which equates to 13-20ppm of free SO2. If sparging, sulfite should also be added to the sparge water before the sparge.
Because sulfite plays such an important role in preventing oxidation, SO2 testing should be utilized to track sulfite consumption. This information can be used to optimize the sulfite usage rate (which most brewers want to minimize). It is suggested to target a 5ppm free SO2 level at the end of the hot side process (up until aeration or oxygenation), which provides a margin of safety without being excessive. Because sulfite acts as a surrogate marker for oxygen exposure, tracking when and how much of it is consumed can help identify procedural areas that need improvement with regard to oxygen exposure.
Short term measures adopted by breweries, to minimize the entry of these aldehydes into fermenting wort, is the addition of carbonyl-complexing agents like potassium metabisulphite (KMS) at the lautering and wort boiling stages. The role of sulphur dioxide (from KMS) in slowing down the formation of stale carbonyl compounds in beer has been widely reported (Nordlo¨r & Winell 1983; Bamforth et al. 1993; Ilett 1995). As current brewing practice is tending towards a clean label, the continued usage of KMS in the future remains doubtful.[36]
The effect of adding sulfite during the mashing has also been examined. Addition of sulfite could potentially give a wort with a high level of polyphenols in different ways. First, it may act as an antioxidant at the early stages of production of beer, thereby preventing oxidation of polyphenols during wort handling, resulting in wort and beer with a high level of proanthocyanidins. Second, sulfite has been shown to reduce and thereby regenerate oxidized flavonoids in model experiments (13).[37] Cites Saucier, C. T.; Waterhouse, A. L. Synergetic activity of catechin and other antioxidants. J. Agric. Food Chem. 1999, 47, 4491-4494.
Adding sulfite to the mash can lower beer haze formation, even when high-oxygen brewing.[37]
It has been suggested that sulfite somehow inhibits an unknown catalyst involved during mashing.[38]
Instructions:
- With the Free SO2 target in mind, the sulfite calculator at FermCalc can help calculate how much sulfite is needed, regardless of what product is being used. Alternately, if you have a particular sodium metabisulfite target in mind, you can use basic math to convert the ppm target to grams (multiply the ppm by the number of liters and divide by 1000).
- Prior to use, weigh the sulfite powder or count and crush the tablets.
- Gently dissolve the sulfite in a small amount of water before adding it to the deoxygenated strike water (and sparge water if applicable). Avoid leaving the sulfite solution sit for an extended time.
- Residual sulfite at the end of the hot-side process needs to be fully neutralized (into sulfate) by using aeration or oxygenation a few minutes before pitching the yeast. Keep in mind that the wort will only increase in dissolved oxygen once all the residual sulfite is neutralized, so in order to provide adequate oxygen to the yeast, a higher amount of aeration or oxygenation will be needed compared to a process that does not use sulfite.
Sulfite at Packaging[edit]
Sulfite may be added when packaging to help delay oxidation during aging/storage.[31][39] Generally, up to 200ppm can be used.[40] It can be used regardless of whether a low-oxygen process of wort production is utilized. Anecdotes suggest that sulfite may even be used when bottle conditioning without any ill effects.[41] However, other home brewers have noted the formation of an off flavor hydrogen sulfide.[42][43][44][45] Sulfite at packaging is rarely used by home brewers, so experience is limited.
Sulfite added to beer at packaging has a tendency to create sulfury aromas, even at very low concentration.[46]
It is well known that sulfite is the direct precursor to hydrogen sulfide, and active yeast are known to create the sulfide in the presence of sulfite. (See hydrogen sulfide for more information.) Given the mixed results experienced by home brewers and the science to understand why, we do not recommend adding sulfite to beer at packaging. Spunding is a better alternative to reducing oxygen exposure, and it is mutually exclusive with adding sulfite at packaging.
Some treatments that inhibit one off-flavor very effectively (e.g., sulfites mask trans-2-nonenal) may enhance other defects (e.g., dimethyltrisulfide increases significantly in the presence of sulfites (9)).[47]
- Effect of the Reducing Power of a Beer on Dimethyltrisulfide Production during Aging
- Antioxidative Mechanisms of Sulfite and Protein-Derived Thiols during Early Stages of Metal Induced Oxidative Reactions in Beer
- Quantification of Protein-Derived Thiols during Atmosphere-Controlled Brewing in Laboratory Scale
- Oxidative Reactions during Early Stages of Beer Brewing Studied by Electron Spin Resonance and Spin Trapping
- Oxygen and Oxygen Radicals in Malting and Brewing: A Review
- Sulfites: Separating Fact from Fiction
- Behaviour of Antioxidants Derived from Hops During Wort Boiling
- Sulfite
- Sulfites in beer: reviewing regulation, analysis and role
- Effects of Sulfur Dioxide and Polyvinylpolypyrrolidone on the Flavor Stability of Beer as Measured by Sensory and Chemical Analysis
Microbial Effects in Beer[edit]
The mechanism of anti-microbial activity of sulfite is entirely dependent on pH because the molecular SO2 form that is responsible for that is only present at low pH. The pH of beer and wort (generally in the range of 3.9-5.5) is too high for reasonable quantities of sulfite to have any anti-microbial effects (particularly on yeast).[48] In other words, sulfite does NOT generally inhibit yeast or bacteria in non-sour beer. (See chemistry and mechanisms below.)
0.8ppm molecular SO2 is generally accepted as the level that starts to inhibit Saccharomyces yeast.[14] to reach this level in a wort at pH 5.0, you would need 1240ppm of free SO2, which equates to 1840ppm sodium metabisulfite.
On rare occasions, home brewers have used sulfite as a way to stabilize wild microbe flavor production in sour beer.[49] In this case, the low pH of sour beer enables the anti-microbial effects of free SO2 by creating more molecular SO2.
In low oxygen brewing, residual sulfite at the time of pitching yeast (that hasn't been adequately neutralized by aeration or oxygenation) has frequently been reported to cause the production of hydrogen sulfide by the yeast and may extend lag time. This is part of why it's important to neutralize all the sulfite around the time of pitching. Aerate/oxygenate either at time of pitching or a couple of minutes beforehand.
When used to remove chlorine/chloramine, the amount of sulfite is very small, and any residual sulfite will oxidize to sulfate long before the yeast is pitched.
Removing Excessive Sulfite from Wine[edit]
Sometimes wine makers have added too much sulfite and need to get rid of it. Excess sulfite can be prevented by following the instructions in this article—most importantly by checking pH, utilizing a sulfite calculator, measuring sulfite levels, and using a quality scale. However, we all make mistakes.
The simplest and low-tech method to remove excess sulfite is to aerate the wine thoroughly, and then leave it open to air (not under airlock) for a day or two. Do this by covering the opening of the vessel with a towel to allow air exchange while keeping out insects. The sulfite will react with oxygen to form sulfate, and also some SO2 will off-gas. Airlock it again when the sulfite aroma/sensation subsides. Unfortunately, this method has a high risk of damaging the wine by oxidation, since the amount of oxygen exposure is not controlled.
Alternately, if you have measured the SO2 level, you may add a controlled amount of hydrogen peroxide (H2O2) to immediately neutralize the desired amount of SO2. To remove 10ppm of free SO2 from wine, add 0.60 mL of 3% hydrogen peroxide per US gallon (this provides a 10% saftey margin).[50][51][52] The H2O2 should be added slowly, with gentle stirring to allow the SO2 species to maintain equilibrium and also to avoid localized oxidation. Again, this method should ONLY be used if you can accurately measure the levels of SO2.
If you find that a bottled wine has too much sulfite (or hydrogen sulfide), using a wine aerator at the time of serving (and then letting it "breathe" for several minutes) is helpful for removing both of these compounds. Serving the wine cold can also be helpful to lessen the volatility (and therefore perception) of these sulfur compounds.
Sulfite Safety[edit]
Legislation in many countries limits the total amount sulfite levels in commercial wine. This may partially be a barrier to prevent commercial wineries from using rotten fruit (which requires a much higher sulfite level than normal). Nevertheless, these limits should be easily attainable for home brewers that avoid using rotten fruit. Sulfite is considered safe in reasonable quantities.[53][31] However pre-fermentation SO2 does increase the production of acetaldehyde by the yeast, so limited use is recommended.
Wines (or other beverages with unlisted ingredients) are required to print on their label that the product "contains sulfites" if the total SO2 detectable in the product is at least 10ppm. This is due to a very small portion of asthmatics that can potentially suffer an asthma attack from the SO2 gas.
Here are some external articles for further reading:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017440/
- https://winefolly.com/tips/sulfites-in-wine/
- https://www.wired.com/2015/06/wine-sulfites-fine-heres-remove-anyway/
- https://www.scientificamerican.com/article/myths-about-sulfites-and-wine/
- https://edis.ifas.ufl.edu/fy731
- https://waterhouse.ucdavis.edu/whats-in-wine/sulfites
Chemistry and Mechanisms[edit]
What article about sulfite would be complete without delving into the chemistry?
Molecular weights for reference:
- Sodium metabisulfite: 190.11 g/mol
- Potassium metabisulfite: 222.32 g/mol
- Sulfur dioxide: 64.066 g/mol
Dissolution[edit]
Metabisulfite salts dissolve in water and rapidly dissociate into ions.
| K2S2O5 + H2O | → | 2 K+ + 2 HSO3- |
|---|---|---|
| [metabisulfite salt] | [metal ions and bisulfite ions] |
Equilibrium[edit]
Three forms of SO2 exist in equilibruim once dissolved. Their ratio depends on pH.
| SO2•H2O | ↔ | H+ + HSO3- | ↔ | 2 H+ + SO32- |
|---|---|---|---|---|
| [molecular SO2] | [hydrogen ion and bisulfite ion] | [hydrogen ions and sulfite ion] |
This figure shows that at most wine and beer pH levels, SO2 is predominantly in the bisulfite ion form, with a higher fraction of molecular SO2 as pH declines.
Mathematically we can calculate the amount of molecular SO2 based on the measured free SO2 and pH:
[Molecular SO2] = [Free SO2] / ( 10(pH - 1.81) + 1 )
Note that the pK1 value for sulfite is generally accepted as 1.81 for wine, but it is affected by the presence of alcohol and other compounds, as well as temperature.
Binding[edit]
When sulfite is added to a wine or beer, a portion of it will bind to compounds in the wine or beer, this is called "bound SO2". The remainder is called "free SO2" and the sum of bound SO2 and free SO2 is called "total SO2". It is the bisulfite ion that binds to compounds, and the bound SO2 no longer contributes to the actions of the forms of free SO2.[14]
Yeast-derived compounds including acetaldehyde, pyruvate, and 2-ketoglutarate represent the majority of substances in wine that bind SO2.[15][54] In sweet wines, unfermented sugar (mainly glucose) causes a significant amount of SO2 binding. SO2 binds to acetaldehyde rapidly and largely irreversibly, but much less quickly to glucose and it does so reversibly. In wines clarified with the help of pectinase, the product of pectin breakdown can significantly contribute to SO2 binding.[55] See Sulphur Dioxide by Ben Rotter for much more detailed information regarding binding.
Sulfite binding to acetaldehyde:
| CH3-CHO + HSO3- | ↔ | CH3-CHOH-SO3- |
|---|---|---|
| [acetaldehyde and bisulfite ion] | [bound compound] |
"Since SO2 will so readily bind with acetaldehyde, thus rendering it ineffective as an antimicrobial and antioxidative agent, it is suggested that acetaldehyde concentrations be kept to the minimum possible. This can be achieved by low [sulfiting] of the must, low pH, low fermentation temperature, and minimal exposure to air."[14]
Adding thiamine is also highly effective for lowering production of yeast-derived compounds that bind SO2.[15] Note that sulfite degrades thiamine, so it should be added during fermentation if sulfite was used pre-fermentation. Thiamine is also destroy by heat.
SO2 binds reversibly to some common phenolic precursors in beer (and wine to a lesser extent), namely caffeic acid and p-coumaric acid. However they are less likely to bind at high temperatures (e.g. during the mash).[14]
It's not possible to predict how much sulfite will become bound.[56] However, we can measure the bound fraction of SO2 by adding a reasonable amount of sulfite, giving it 4 to 5 days to reach equilibrium, and then testing both the free SO2 and the total SO2 (see SO2 testing). The difference between free SO2 and total SO2 will be the amount that is bound. This still isn't useful for predicting how much will become bound if more sulfite is added because the relationship between bound SO2 and free SO2 is non-linear -- a smaller proportion of SO2 becomes bound as the amount of total SO2 increases.
However these general guidelines exist for helping to predict SO2 binding in fermented wine:
- 50% of total SO2 up to 50ppm will bind.
- 30% of additional SO2 added between 50-100ppm total SO2 will bind.
- Binding will be minimal for additional SO2 added beyond 100ppm total SO2.
[We need an example, or better yet, a calculator]
Oxidation Prevention[edit]
- Bisulfite ion irreversibly inactivates polyphenol oxidase and other oxidation enzymes (e.g. tyrosinase and laccase), and thus prevents browning of wines.[57][58][14] This is greatly significant because enzymatic oxidation occurs incredibly faster than direct chemical oxidation. When pressing fruit for wine, sulfite needs to be added to the must immediately from the start of pressing (or earlier e.g. crushing or maceration) to inhibit enzymatic oxidation. These enzymes lose their activity during fermentation.
- Acetaldehyde gives wine an oxidized flavor/aroma. When bound with sulfite it is no longer aromatic, so its perception is significantly reduced, resulting in a more fresh tasting wine (or beer).[15]
- When SO2 binds to certain compounds (polyphenols/catechols), it prevents those molecules from reacting with oxygen to form hydrogen peroxide.
- Free sulfite ion reacts directly with oxygen (slowly, and this reaction is probably negligible in wine, although it's plausible that this reaction occurs with greater speed at higher pH). However, all forms of SO2 react readily with hydrogen peroxide (H2O2) and other Reactive Oxygen Species.[59]
Reaction with oxygen (theoretical):
| 2 HSO3- + O2 | → | 2 H+ + 2 SO42- |
|---|---|---|
| [bisulfite ion and oxygen] | [acid and sulfate ion] |
Reaction with hydrogen peroxide:
| HSO3- + H2O2 | → | H+ + SO42- + H2O |
|---|---|---|
| [bisulfite and hydrogen peroxide] | [acid, sulfate, and water] |
Because oxygen can react with different substances in wine (e.g. catechols, quinones, ascorbic acid, etc.), between 1-2 moles of SO2 is consumed for every 1 mol of O2 dissolved in wine. The ratio of SO2 to O2 consumed is typically around 1.7:1.[14][60][61] Note that in fermented wine (after the oxidation enzymes have been inactivated), that both dissolved oxygen and sulfite can exist simultaneously in the wine for some amount of time (hours to days, depending on the level of oxygen exposure).
Much less is known about the chemistry of SO2 in beer.
Anti-microbial Activity[edit]
- The mechanism of action is not entirely clear, but SO2 is likely to disrupt intracellular pH, and disrupt the structure of proteins including enzymes and DNA.[25]
- Molecular SO2 inhibits microbes even in relatively low concentrations (e.g. 0.4-0.8ppm) and in higher concentrations (e.g. 0.8-1.2ppm) it can destroy some of the susceptible microbes in a wild culture. Bisulfite ions and sulfite ions generally do not have a significant anti-microbial effect because they do no enter the cell.[62]
- Bound SO2 does not affect yeast but it has a weak anti-bacterial effect, which is significant because there is typically a much higher concentration of bound SO2 relative to molecular SO2.[14] This is because bacteria consume acetaldehyde, which subsequently releases the SO2.
While the molecular SO2 is rapidly taken up by microbes, cell death (for susceptible strains) still may take up to 24 hours to occur.
Effect on Color[edit]
Wines fermented with sulfite are shown to better retain color, although the mechanism seems to be unknown. The effect of sulfite on aiding color extraction appears to be negligible at reasonable doses, as well as the effect of sulfite on bleaching wine.
Sulfite Loss[edit]
The total SO2 in the wine, beer, juice/must, or wort is not the total amount that was added because some of it escapes or reacts to form other compounds.
Sulfite may be lost through:
- Volatilization of molecular SO2, especially if the must/wort/etc. is agitated, and during CO2 release during fermentation. This may also occur to a small degree through barrel walls and bottle corks.
- Being consumed from oxidative reactions, including reactions with oxygen, hydrogen peroxide, quinones, or previously oxidized phenolic compounds in the wine/etc reestablishing equilibrium.
Microbial Resistance[edit]
Microbes (particularly yeast) frequently exposed to sulfite (e.g. in wine barrels) can develop permanent sulfite resistance. Because the mechanism of action is concentration-dependent, it is therefore important to use a smaller larger number of large doses during wine aging, rather than a larger number of small doses.
Some microbiologists speculate that acetaldehyde production is a primary mechanism used by microbes to resist the effects of SO2.[25] Increased pumping of sulfite out of the cell may be another mechanism.[63]
Reactions with Halogens and Chloramine[edit]
Sulfite reacts directly with chlorine (e.g. in tap water) and iodine (e.g. in a Ripper test).
| SO2 + 2 H2O + I2 | → | H2SO4 + 2 HI |
|---|---|---|
| [sulfite, water, and iodine] | [sufuric acid and hydroiodidic acid] |
Reaction with chlorine (in the form of hypochlorous acid):[64]
| HSO3- + HOCl | → | SO42- + 2 H+ + Cl- |
|---|---|---|
| [sulfite and hypochlorous acid] | [sulfate, hydrogen ion, and chloride] |
Reaction with chloramine:[65][66]
| SO2 + 2 H2O + NH2Cl | → | 2 H+ + SO42- + Cl- + NH4+ |
|---|---|---|
| [sulfite, water, and chloramine] | [hydrogen ion, sulfate, chloride, and ammonium] |
Note that these products will dissociate and react with substances in the water/beer/wine and/or be consumed.
Additional Resources[edit]
- SO2 testing: Methods to measure Free or Total SO2
External sites:
- Sulphur Dioxide (Rotter, 2011): Well-referenced and thorough analysis of SO2 chemistry, with some practical advice.
Potential sources
- Catechols in beer https://onlinelibrary.wiley.com/doi/full/10.1111/1541-4337.12352
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC168295/
- "Investigations of the Protective Mechanism of Sulfite Against Beer Staling and Formation of Adducts with Trans-2-Nonenal"
- Sensing free sulfur dioxide in wine.
- Sulfur dioxide acute exposure guideline levels
- http://www.wineanorak.com/mercaptansinwine.htm
- https://www.internationalwinechallenge.com/Canopy-Articles/struck-match-in-chardonnay-whats-it-all-about.html
- https://pubs.acs.org/doi/10.1021/jf020756c#
- Sulfites for Oxygen Control
- http://www.themodernbrewhouse.com/wp-content/uploads/2016/11/Sulfites-in-beer-reviewing-regulation-analysis-and-role.pdf Sulfites in Beer: Reviewing Regulation, Analysis and Role
- Kaneda, H., Takashio, M., Osawa, T., Kawakishi, S., and Tamaki, T. (1996) Behavior of sulfites during fermentation and storage of beer, J. Am. Soc. Brew. Chem. 54, 115–120.
- Andersen, M. L., Outtrup, H., and Skibsted, L. H. (2000) Potential antioxidants in beer assessed by esr spin trapping, J. Agric. Food Chem. 48, 3106–3111.
- Duan, W., Roddick, F. A., Higgins, V. J., and Rogers, P. J. A parallel analysis of H2S and SO2 formation by brewing yeast in response to sulfur-containing amino acids and ammonium ions. J. Am. Soc. Brew. Chem. 62:35-41, 2004.
References[edit]
- ↑ https://www.therealreview.com/2018/07/17/understanding-hydrogen-sulphide-and-sulphur-dioxide/
- ↑ Williamson, B. "Recognizing Wine Flaws." Williamson Wines. Accessed online March 2020.
- ↑ Mansfield, AK. "Kicking up a Stink: Treatment for Sulfur Off-Odors." Cellar Dweller. Cornell University - NYSAES. April 2010.
- ↑ a b c d Baert JJ, De Clippeleer J, Hughes PS, De Cooman L, Aerts G. On the origin of free and bound staling aldehydes in beer. J Agric Food Chem. 2012;60(46):11449–11472.
- ↑ Zufall C, Tyrell Th. The influence of heavy metal ions on beer flavour stability. J Inst Brew. 2008;114(2):134–142.
- ↑ a b c Lund MN, Andersen ML. Detection of Thiol Groups in Beer and Their Correlation with Oxidative Stability. J Am Soc Brew Chem. 2011;69(3):163–169.
- ↑ Sodium Metabisulfite MSDS Ineos.
- ↑ "Potassium Metabisulfite Aging." winemakingtalk.com. 2012.
- ↑ "Potassium metabisulfite." Pubchem.
- ↑ https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01504-en.pdf
- ↑ Kraus, Ed. "How To Tell If Your Potassium Metabisulfite Is Old." Wine Making Blog.
- ↑ "Technical Information Sheet: Potassium Metabisulphite - Preservatives." Murphy & Son, Ltd.
- ↑ "Recommended Shelf Life." Romil Pure Chemistry.
- ↑ a b c d e f g h i j k l Rotter, Ben. "Sulphur Dioxide." Improved Winemaking. 2011.
- ↑ a b c d Werner, M., Rauhut, D., Cottereau, P. "Yeasts and Natural Production of Sulphites." Internet Journal of Enology and Viticulture. 2009 N12/3
- ↑ a b c Lund MN, Petersen MA, Andersen ML, Lunde C. Effect of protease treatment during mashing on protein-derived thiol content and flavor stability of beer during storage. J Am Soc Brew Chem. 2015;73(3):287–295.
- ↑ a b Golston AM. The impact of barley lipids on the brewing process and final beer quality: A mini-review. Tech Q Master Brew Assoc Am. 2021;58(1):43–51.
- ↑ Mikyška A, Hrabak M, Hašková D, Šrogl J. The role of malt and hop polyphenols in beer quality, flavour and haze stability. J Inst Brew. 2002;108(1):78–85.
- ↑ Pascoe HM, Ames JM, Chandra S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J Am Soc Brew Chem. 2003;61(4):203–209.
- ↑ Kunz T, Brandt NO, Seewald T, Methner FJ. Carbohydrates addition during brewing – effects on oxidative processes and formation of specific ageing compounds. BrewingScience. 2015;68(7):78–92.
- ↑ Callemien D, Collin S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—a review. Food Rev Int. 2009;26(1):1–84.
- ↑ Bamforth CW, Lentini A. The flavor instability of beer. In: Bamforth CW, ed. Beer: A Quality Perspective. Academic Press; 2009:85–109.
- ↑ Martinez-Periñan E, Hernández-Artiga MP, Palacios-Santander JM, ElKaoutit M, Naranjo-Rodriguez I, Bellido-Milla D. Estimation of beer stability by sulphur dioxide and polyphenol determination. Evaluation of a Laccase-Sonogel-Carbon biosensor. Food Chem. 2011;127(1):234–239.
- ↑ a b c Andersen ML, Outtrup H, Skibsted LH. antioxidants in beer assessed by ESR spin trapping. J Agric Food Chem. 2000;48(8):3106–3111.
- ↑ a b c d Lea, Andrew. "Sulphur Dioxide - the Cidermaker's Friend." 2011.
- ↑ Jolicoeur, Claude. The New Cider Maker's Handbook: A Comprehensive Guide for Craft Producers. Chapter 14, section 1. 2013.
- ↑ Martin, Valentina, et al. "Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review." Fermentation. 2018, 4, 76; doi:10.3390/fermentation4030076
- ↑ a b Pambianchi, Daniel. "A Review of Sulfite Management Protocols Based on SO2 Levels and Type of Wine." Techniques in Home Winemaking. 2014.
- ↑ a b c Kunz T, Reinhardt Ch, Eon-Jeong L, Dörr T, Radowski A, Methner FJ. Impact of fermentable and non fermentable carbohydrates on the sweetness, improvement of palate fullness and SO2-content in beer. BrewingScience. 2012;65(11):140–149.
- ↑ Rabe, Bryan. "METHODS OF THE LOW OXYGEN BREWHOUSE." LowOxygenBrewing.com
- ↑ a b c Guido, Luis. "Sulfites in beer: reviewing regulation, analysis and role." Sci. agric. (Piracicaba, Braz.) vol.73 no.2 Piracicaba Mar./Apr. 2016
- ↑ a b Zhao H, Li H, Sun G, Yang B, Zhao M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J Sci Food Agric. 2013;93(4):910-917.
- ↑ Yang D, Gao X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci Technol. 2021;110:754-764.
- ↑ Lewis MJ, Bamforth CW. Chapter 12: Oxygen. In: Lewis MJ, Bamforth CW, eds. Essays in Brewing Science. Springer; 2006:131–142.
- ↑ Wu MJ, Rogers PJ, Clarke FM. 125th anniversary review: The role of proteins in beer redox stability. J Inst Brew. 2012;118(1):1–11.
- ↑ EtokAkpan OU. Preliminary study of fat oxidation in sorghum and maize brewing. World J Microbiol Biotechnol. 2004;20:569–573.
- ↑ a b Andersen ML, Skibsted LH. Modification of the levels of polyphenols in wort and beer by addition of hexamethylenetetramine or sulfite during mashing. J Agric Food Chem. 2001;49(11):5232–5237.
- ↑ Chapon L, Chemardin M. The dissolving and oxidation of malt tannoids on mashing-in. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1964;22(1):244–258.
- ↑ Siqueira, PB, et al. "O Processo De Fabricação Da Cerveja E Seus Efeitos Na Presença De Polifenóis (The Beer Manufacturing Process And Its Effects On The Presence Of Polyphenols)" Alimentos e Nutrição Araraquara, vol. 19, no. 4, 2008, pp. 491-498.
- ↑ Kunze, Wolfgang. Technology Brewing & Malting. Edited by Olaf Hendel, 6th English Ed., VBL Berlin, 2019. p. 509.
- ↑ Huolihan, Jake. "Post-Fermentation Oxidation: The Impact Adding Sodium Metabisulfite at Packaging has on Beer." Brulosophy. 2019
- ↑ Sodium metabisulfite. HomebrewTalk forum. 2020. Accessed July 2020.
- ↑ http://brulosophy.com/2020/04/06/impact-higher-dosage-rates-of-sodium-metabisulfite-smb-have-on-beer-character-exbeeriment-results/
- ↑ https://www.homebrewtalk.com/threads/perceived-flavor-threshold-sodium-metabisulfite-smb-and-potassium-metabisulfite-campden.670439/page-2#post-8908000
- ↑ https://www.homebrewtalk.com/threads/sulfur-flavor-in-beer.698775/
- ↑ Nielsen H. The control of oxygen in beer processing. J Inst Brew. 1973;79(2):147–154.
- ↑ Callemien D, Dasnoy S, Collin S. Identification of a stale-beer-like odorant in extracts of naturally aged beer. J Agric Food Chem. 2006;54(4):1409–1413.
- ↑ King, AD Jr., et al. Factors Affecting Death of Yeast by Sulfur Dioxide." Journal of Food Protection. Vol. 44, No. 2, pp 92-97 February 1981.
- ↑ Tonsmeire, Michael. "Courage Russian Imperial Stout." The Mad Fermentationist. 2007.
- ↑ "Technical Note 06—Removal from and addition of sulfur dioxide to must, juice and wine." AWRI Technical services group, 2007.
- ↑ Jackisch, Philip. Modern Winemaking. Cornell University Press. 1985. p 94.
- ↑ Henderson, P. "Sulfur Dioxide & Wine Additives." Introduction to Enology lecture, 2014.
- ↑ "Sulfites - USA." Institute of Agriculture and Natural Resources, Food Allergy Research and Resource Program.
- ↑ Jackowetz, J.N., and Mira de Orduña, R. "Survey of SO2 binding carbonyls in 237 red and white table wines." Food Control. Volume 32, Issue 2, August 2013. Pages 687-692.
- ↑ Lea, A. et al. "Analytical techniques for the estimation of sulphite binding components in ciders and wines." International Journal of Food Science and Technology. 2000, 35, 105-112.
- ↑ Lea, A, and Jarvis, B. "Sulphite binding in ciders." International Journal of Food Science and Technology. 2000. 35, 113-127.
- ↑ Embs, R.J. and Markakis, P. "The Mechanism of Sulfite Inhibition of Browning Caused by Polyphenol Oxidase." Journal of Food Science. 30: 753-758. doi:10.1111/j.1365-2621.1965.tb01836.x. 1965.
- ↑ Sayavedra-Soto, L.A. and Montgomery, M.W. "Inhibition of Polyphenoloxidase by Sulfite." Journal of Food Science. 51: 1531-1536. doi:10.1111/j.1365-2621.1986.tb13852.x 1986.
- ↑ https://www.apps.fst.vt.edu/extension/enology/EN/133.html
- ↑ "Impact of Oxygen on Quality of White Wine." 2013
- ↑ Danilewicz, John. "Reaction of Oxygen and Sulfite in Wine." Am J Enol Vitic. January, 2016 67: 13-17.
- ↑ Stratford, M. and Rose, A. "Transport of Sulphur Dioxide by Saccharomyces cerevisiae." Journal of General Microbiology. 1986. 132, 1-6.
- ↑ Zimmer A., et al. "QTL Dissection of Lag Phase in Wine Fermentation Reveals a New Translocation Responsible for Saccharomyces cerevisiae Adaptation to Sulfite." PLoS ONE. 9(1): e86298. 2014.
- ↑ Jolley, RL, and Carpenter, JH. "Aqueous Chemistry of Chlorine: Chemistry, Analysis, and Environmental Fate of Reactive Oxidant Species." 1982. doi:10.2172/5505533.
- ↑ Yiin, Boudin, et al. "Nonmetal redox kinetics: general-acid-assisted reactions of chloramine with sulfite and hydrogen sulfite." Inorg. Chem. 1987, 26, 21, 3435-3441.
- ↑ "Experiments in Removing Chlorine and Chloramine From Brewing Water" 1998