Ferulic acid: Difference between revisions
No edit summary Tags: Mobile edit Mobile web edit |
No edit summary |
||
| (32 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:Ferulic-acid.png|thumb|Ferulic acid structure]] | |||
Ferulic acid is one of the main [[phenolic compounds|phenolic]] acids in [[barley]], [[wheat]], and [[rye]] grains (365 to 605 µg/g in barley).<ref name=egi/> It is an effective [[antioxidants|antioxidant]], it retards the degradation of iso-α-acids, and it is a potent UV light absorber.<ref name=habkos>Habschied K, Košir IJ, Krstanović V, Kumrić G, Mastanjević K. [https://www.mdpi.com/2306-5710/7/2/38 Beer polyphenols—bitterness, astringency, and off-flavors.] ''Beverages.'' 2021;7(2):38.</ref><ref name=bsp/><ref name=Siqueira/><ref>Graf E. [https://www.sciencedirect.com/science/article/abs/pii/089158499290184I Antioxidant potential of ferulic acid.] ''Free Radic Biol Med.'' 1992;13(4):435–448.</ref> Ferulic acid exists mainly ester-bound to [[Beta-glucans and arabinoxylans|arabinoxylans]]; only a minor part of ferulic acid is present in malts in free forms.<ref name=Siqueira>Siqueira PB, Bolini H, Macedo GA. [https://www.researchgate.net/publication/49599952_O_PROCESSO_DE_FABRICACAO_DA_CERVEJA_E_SEUS_EFEITOS_NA_PRESENCA_DE_POLIFENOIS O processo de fabricação da cerveja e seus efeitos na presença de polifenóis. (The beer manufacturing process and its effects on the presence of polyphenols.)] ''Alimentos e nutrição.'' 2008;19(4):491–498.</ref> Some additional ferulic acid can be released from the arabinoxylans by [[enzymes]] during [[mashing]].<ref name=Szwajgier>Szwajgier D. [https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2011.tb00505.x Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts.] ''J Inst Brew.'' 2011;117(4):569–577.</ref><ref name=bsp>Briggs DE, Boulton CA, Brookes PA, Stevens R. [[Library|''Brewing Science and Practice.'']] Woodhead Publishing Limited and CRC Press LLC; 2004.</ref><ref name=egi>Egi A, Speers RA, Schwarz PB. [https://www.mbaa.com/publications/tq/tqPastIssues/2004/Abstracts/0803-01.htm Arabinoxylans and their behavior during malting and brewing.] ''Tech Q Master Brew Assoc Am.'' 2004;41(3):248–267.</ref><ref name=vanvan/><ref name=wangas/><ref name=zhao>Zhao H. [https://www.sciencedirect.com/science/article/pii/B9780124046993000640 Chapter 64: Effects of processing stages on the profile of phenolic compounds in beer.] In: Preedy V, ed. ''Processing and Impact on Active Components in Food.'' Academic Press; 2015:533-539.</ref><ref name=sibpla>Šibalić D, Planinić M, Jurić A, Bucić-Kojić A, Tišma M. [https://link.springer.com/article/10.1007/s11696-020-01276-1 Analysis of phenolic compounds in beer: from raw materials to the final product.] ''Chem Zvesti.'' 2021;75(1):67–76.</ref> Release is driven by cinnamoyl esterase, an enzyme that is most effective during an extended rest at 40–45°C and pH 5.2–6.6 with stirring.<ref name=cargui/><ref name=wangas>Wannenmacher J, Gastl M, Becker T. [https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/1541-4337.12352 Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality.] ''Compr Rev Food Sci Food Saf.'' 2018;17(4):953–988.</ref><ref name=vanvan/><ref name=schwarz/> Enzymatic release declines with increasing temperature up to about 65°C where there is none; at this point the amount of free ferulic acid extracted is solely dependent on the free form created during malting.<ref name=vanvan/><ref name=cargui>Carvalho DO, Guido LF. [https://www.sciencedirect.com/science/article/abs/pii/S0308814621020999 A review on the fate of phenolic compounds during malting and brewing: technological strategies and beer styles.] ''Food Chem.'' 2022;372:131093.</ref><ref name=schwarz>Schwarz KJ, Boitz LI, Methner FJ. [https://www.tandfonline.com/doi/abs/10.1094/ASBCJ-2012-1011-02 Release of phenolic acids and amino acids during mashing dependent on temperature, pH, time, and raw materials.] ''J Am Soc Brew Chem.'' 2012;70(4):290–295.</ref> Ferulic acid level increases during [[boiling]] as a result of extraction from hops as well as water evaporation, despite some being converted to 4VG (see below).<ref name=vanvan/> The concentration of ferulic acid remains stable during [[fermentation]] (with typical brewers [[yeast]]), but then declines during storage.<ref name=szwpie>Szwajgier D, Pielecki J, Targoński Z. [https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.2050-0416.2005.tb00222.x The release of ferulic acid and feruloylated oligosaccharides during wort and beer production.] ''J Inst Brew.'' 2005;111(4):372–379.</ref> | |||
Ferulic acid | Ferulic acid itself is generally flavorless, having a flavor threshold in beer as high as 600 ppm.<ref name=vanvan/> However, it is notable as a precursor to the more flavor-active 4-vinyl guaiacol (4VG). Ferulic acid is transformed into 4VG by decarboxylation, which occurs during boiling to a small extent, but mostly by the enzymes present in many wild microbes and certain strains of brewers yeast, deemed phenolic off-flavor positive (POF+).<ref name=bsp/><ref name=vansai>Vanbeneden N, Saison D, Delvaux F, Delvaux FR. [https://pubs.acs.org/doi/abs/10.1021/jf8019453 Decrease of 4-vinylguaiacol during beer aging and formation of apocynol and vanillin in beer.] ''J Agric Food Chem.'' 2008;56(24):11983–11988.</ref> 4VG gives a spicy clove flavor that is usually undesirable, but is crucial to the flavor profile of some specialty beers. The flavor threshold of 4VG in blond specialty beers is quite low, 0.37 ppm.<ref name=vanvan>Vanbeneden N, Van Roey T, Willems F, Delvaux F, Delvaux FR. [https://www.sciencedirect.com/science/article/abs/pii/S0308814608003348 Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing.] ''Food Chem.'' 2008;111(1):83–91.</ref> | ||
Ferulic acid is | |||
The flavor threshold of 4VG in blond specialty beers is | |||
==See also== | ==See also== | ||
*[[Phenolic compounds]] | *[[Phenolic compounds]] | ||
*[[Weissbier]] | |||
==References== | ==References== | ||
Latest revision as of 13:20, 3 April 2024
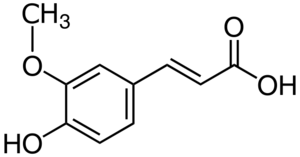
Ferulic acid is one of the main phenolic acids in barley, wheat, and rye grains (365 to 605 µg/g in barley).[1] It is an effective antioxidant, it retards the degradation of iso-α-acids, and it is a potent UV light absorber.[2][3][4][5] Ferulic acid exists mainly ester-bound to arabinoxylans; only a minor part of ferulic acid is present in malts in free forms.[4] Some additional ferulic acid can be released from the arabinoxylans by enzymes during mashing.[6][3][1][7][8][9][10] Release is driven by cinnamoyl esterase, an enzyme that is most effective during an extended rest at 40–45°C and pH 5.2–6.6 with stirring.[11][8][7][12] Enzymatic release declines with increasing temperature up to about 65°C where there is none; at this point the amount of free ferulic acid extracted is solely dependent on the free form created during malting.[7][11][12] Ferulic acid level increases during boiling as a result of extraction from hops as well as water evaporation, despite some being converted to 4VG (see below).[7] The concentration of ferulic acid remains stable during fermentation (with typical brewers yeast), but then declines during storage.[13]
Ferulic acid itself is generally flavorless, having a flavor threshold in beer as high as 600 ppm.[7] However, it is notable as a precursor to the more flavor-active 4-vinyl guaiacol (4VG). Ferulic acid is transformed into 4VG by decarboxylation, which occurs during boiling to a small extent, but mostly by the enzymes present in many wild microbes and certain strains of brewers yeast, deemed phenolic off-flavor positive (POF+).[3][14] 4VG gives a spicy clove flavor that is usually undesirable, but is crucial to the flavor profile of some specialty beers. The flavor threshold of 4VG in blond specialty beers is quite low, 0.37 ppm.[7]
See also[edit]
References[edit]
- ↑ a b Egi A, Speers RA, Schwarz PB. Arabinoxylans and their behavior during malting and brewing. Tech Q Master Brew Assoc Am. 2004;41(3):248–267.
- ↑ Habschied K, Košir IJ, Krstanović V, Kumrić G, Mastanjević K. Beer polyphenols—bitterness, astringency, and off-flavors. Beverages. 2021;7(2):38.
- ↑ a b c Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b Siqueira PB, Bolini H, Macedo GA. O processo de fabricação da cerveja e seus efeitos na presença de polifenóis. (The beer manufacturing process and its effects on the presence of polyphenols.) Alimentos e nutrição. 2008;19(4):491–498.
- ↑ Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13(4):435–448.
- ↑ Szwajgier D. Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J Inst Brew. 2011;117(4):569–577.
- ↑ a b c d e f Vanbeneden N, Van Roey T, Willems F, Delvaux F, Delvaux FR. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008;111(1):83–91.
- ↑ a b Wannenmacher J, Gastl M, Becker T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr Rev Food Sci Food Saf. 2018;17(4):953–988.
- ↑ Zhao H. Chapter 64: Effects of processing stages on the profile of phenolic compounds in beer. In: Preedy V, ed. Processing and Impact on Active Components in Food. Academic Press; 2015:533-539.
- ↑ Šibalić D, Planinić M, Jurić A, Bucić-Kojić A, Tišma M. Analysis of phenolic compounds in beer: from raw materials to the final product. Chem Zvesti. 2021;75(1):67–76.
- ↑ a b Carvalho DO, Guido LF. A review on the fate of phenolic compounds during malting and brewing: technological strategies and beer styles. Food Chem. 2022;372:131093.
- ↑ a b Schwarz KJ, Boitz LI, Methner FJ. Release of phenolic acids and amino acids during mashing dependent on temperature, pH, time, and raw materials. J Am Soc Brew Chem. 2012;70(4):290–295.
- ↑ Szwajgier D, Pielecki J, Targoński Z. The release of ferulic acid and feruloylated oligosaccharides during wort and beer production. J Inst Brew. 2005;111(4):372–379.
- ↑ Vanbeneden N, Saison D, Delvaux F, Delvaux FR. Decrease of 4-vinylguaiacol during beer aging and formation of apocynol and vanillin in beer. J Agric Food Chem. 2008;56(24):11983–11988.