Phenolic compounds
Phenolic substances (sometimes called tannins or polyphenols) are integral and abundant components of plant cell walls, where they serve as a natural defense system against microbial pathogens, insects, and herbivores, as well as reducing oxidative stress.[1][2][3][4] During the brewing process, a wide and complex array of phenolic compounds from grain and hops are released into the wort and therefore are always present in beer. These compounds play a key role in beer quality since they directly influence the flavor, color, and clarity of beer.[1][5] The effects of these compounds are closely related to the amount of oxidation that occurs. Phenolic compounds readily scavenge oxygen radicals and therefore serve as natural antioxidants, helping to protect grain and hop flavor compounds from oxidation, thereby improving flavor stability. As antioxidants, they also provide a health benefit when consuming beer and wine. However, as the phenolic compounds oxidize during brewing or beer storage, they add a darker color, add haze, and contribute a variety of off-flavors including astringency and harsh bitterness. When modified by certain types of yeast or bacteria, phenolic compounds can add distinct flavors such as spicy clove, pepper, or smoke. These types of flavors are usually undesirable and are generally referred to as phenolic off-flavor (POF). Despite this term, these flavors are an important part of some specialty beer styles such as weissbier and saison.
The phenolic content of beer depends largely on brewing practice and raw materials.[6][1][7] Beer exhibiting high phenolics content (and the related increase in antioxidant activity) displays better flavor quality, more stable flavor and aroma, improved foam stability, and longer shelf life (i.e. improved clarity) as compared with beer with less phenolics.[8] Due to these beneficial effects, interest is shifting towards preserving the phenolic compounds from the raw materials to the final beer.[1][9][10][11][12][13][14] With this in mind, brewers have numerous ways to improve the composition of phenolic compounds and the level of antioxidant activity in the finished beer.[2][15] Perhaps most importantly, brewers may choose to minimize oxygen exposure during the brewing process, resulting in much greater natural antioxidant activity in the final beer while avoiding the negative effects of phenolic compounds. Various other production parameters also have a major impact on the level of phenolic compounds, such as milling intensity, mashing temperature(s), PVPP treatment, and filtration, which can all affect the amount extracted from the malt and carried through to the beer.[16] The choice and quantity of raw materials (including malt, hops, and tannin additives) is also important because different ingredients will contribute varying levels of phenolic compounds to the wort.[17] Lastly, beer storage and packaging conditions can have a significant impact on the structure of phenolic compounds, which may lead to haze formation or changes to flavor.[15]
Structure and classification[edit]
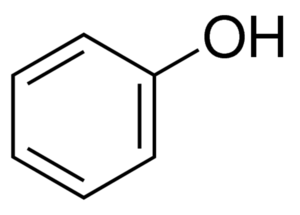
The phenolic unit is an aromatic ring connected to a hydroxyl (OH) group, and this phenolic ring is connected other structural elements to form a variety of phenolic compounds.[6] They constitute a very diverse group of substances whose components vary greatly in chemical structure and therefore differ in reactivity.[11] Phenolic compounds exist not only individually, but also in bound form attached to other molecules such as esters, sugars (glycosides), and organic acids.[18][19][20][15] Barley contains hundreds of different phenolic compounds[15] and the structural classification is very complex,[21][22] and therefore discussion here will be limited.
Phenolic acids and simple phenols[edit]
Phenolic acids are among the most abundant phenolic compounds in beer.[11] Structurally they contain a single phenolic ring structure along with a carboxylic acid group. Phenolic acids are divided into: benzoic acid derivatives and cinnamic acid derivatives.[16] Most phenolic acids (the cinnamic acid derivatives in particular) in malt and beer are bound to cell wall polysaccharides, amides (i.e. phenolamides), or other molecules.[11][15][1][23][24][25] Volatile phenols are formed when the carboxylic acid group is removed from phenolic acids.[1] The aromatic alcohol tyrosol is formed by fermentation of the amino acid tyrosine, and is present in beer in significant concentrations.[22]
| Benzoic acid derivatives | Cinnamic acid derivatives |
|---|---|
|
|
-
Ferulic acid
Polyphenols[edit]
Another main group of phenolic compounds in beer can be broadly termed as "polyphenols", which contain multiple phenolic rings.[26] These consist of both flavonoids (e.g. flavanols, flavones, flavonones, flavonols, and proanthocyanidins) and non-flavonoids (e.g. stilbenes, chalcones, lignans, and hydrolyzable tannins).[16][27][1][22] Flavonoids (also called anthocyanogens) are the major free phenolic compounds in barley,[15] and the main groups of free flavonoids are monomer flavanols (flavan-3-ols) and polymeric flavanols (proanthocyanidins AKA condensed tannins).[1][11] For example, catechin (flavan-3-ol monomer) is an abundant free polyphenolic compound in both barley and hops.[16][19][1][27] Catechin frequently is a component of the over 50 different proanthocyanidins detected in barley grains, including the dimers prodelphinidin B3 and procyanidin B3.[28][29] Kaempferol and quercetin glycosides and their malonyl esters are notable examples of flavonols extracted from hops.[1][27][6] Prenylflavonoids, also found in hops, represent a class of flavonoids with at least one prenyl or geranyl group attached to the phenolic ring.[27][30] Multifidol and multifidol glucosides are other constituents of hops, which can have significant flavor impact even at very low concentration.[27]
Amino phenols[edit]
Finally, wort and beer contains amino phenols in the form of phenolic amino acids, which can also be part of peptides or proteins.[11][15][31] The other types phenolic compounds are derived from these phenolic amino acids, specifically phenylalanine and to a lesser extent tyrosine (via the Ehrlich pathway).[1]
Sources of phenolic compounds[edit]
All phenolic compounds originate from plant material, which for beer typically includes grains and hops.[1][2] Other plant ingredients such as fruit (i.e. in fruit beer) will contribute phenolic compounds if they are used as ingredients. In lightly-hopped industrial beers, barley malt contributes 70–80% of the total polyphenols in beer, with the remainder coming from hops.[1] However, in the more hoppy styles that are popular among craft and home brewers, much more of the beers' phenolic content is contributed by the hops.[16][12][32] Levels of phenolic content in wort and beer can vary widely.[32][9] The final composition of phenolic compounds in beer depends on the characteristics of the raw materials used and the methods of brewing and fermentation.[20]
Grain[edit]
During mashing, phenolic compounds are extracted into wort from the grain. Cereal grain contains phenolic acids and a variety of polyphenols, making up around 0.2% of grain by dry weight.[16] In barley, the phenolic compounds are predominantly found in the outer layers of the grain (husk, pericarp, testa, and aleurone cells), while their concentration is noticeably lower in the endosperm.[1][33][34] The total amount of phenolic compounds depends on the genetic variety and growing conditions, although the relative amounts are fairly consistent.[1][16][15][2][35][33][36] The total extractable phenolic content (and antioxidant potential) increases during malting.[16][1][15][27][37][35][38][39][40][41][42][43][3]
The phenolic acids ferulic acid, p-coumaric acid, gallic acid, caffeic acid, vanillic acid, and sinapinic acid are among the main phenolic compounds in malt.[16][1][4][2][44][40][45][46][47] Most of the phenolic acids in cereals are insolubly bound to cell wall carbohydrates (arabinoxylans), while some are bound in the form of glycosides or are in free form and thus more easily extractable.[1][16][44][2] The higher phenolic content in malt has been mainly attributed to the enzymatic release of bound phenolics during malting.[16] Optimization of the malting process can protect the polyphenols present in barley and also promote the formation of new antioxidant compounds.[35][20][48][16]
Wheat and rye contribute somewhat less total phenolic compounds compared to barley,[49][50][51] while oats and buckwheat contribute the lowest amount of phenolics, much lower than barley.[51]
Factors affecting phenolic compound extraction from grain:
- Malt parameters - More phenolics are extracted from well-modified malts during mashing.[5][35][52][53] Vienna malt, melanoidin malt, and smoked malt provide more phenolic content than other base malts.[49] Malt color and the use of specialty malts generally has a negligible effect on phenolic acid content of the resulting wort.[24][4] Smaller kernels generally contain higher levels of phenolics.[33] Phenolic acid content is positively correlated positively with the Kolbach index and soluble nitrogen content, and is negatively related to malt extract and viscosity.[1]
- Adjuncts - Use of unmalted cereal adjuncts (maize, wheat, oats, rice, buckwheat, rye, millet, and barley) results in significantly lower levels of free phenolic acids (Congress mashing).[24][7] For example, wort produced with 50% unmalted wheat flakes contains 15–43% less free ferulic acid compared to wort with 100% malted barley.[24] There are two exceptions to this: rice increases p-coumaric acid and rye does not alter the ferulic acid concentration.
- Milling - Fine milling (causing increased husk damage) increases the amount of phenolic compounds in the wort.[1][54][33] Grain conditioning will decrease the total phenolic content in wort, due to higher husk integrity.[1][27]
- Ferulic acid rest - Phenolic acid extraction can be increased by enzymatic activity during mashing.[24][1][15][12] Release of bound phenolic acids is driven by cinnamoyl esterase (also called feruloyl esterase or ferulic acid esterase), an enzyme with maximal activity at 40–45°C (with an extended rest) and pH 5.2–6.6.[16][1][24][4] Enzymatic release declines with increasing temperature up to about 65°C where there is none; at this point the amount of phenolic acid extracted is solely dependent on the free form created during malting.[24][16]
- Mash temperature - Besides conducting a ferulic acid rest, the total phenolic content tends to increase with increasing mash temperature up to around 70°C where it peaks (mash-out temperature causes a decrease).[1][35][5][53][16] The decrease at high temperatures is likely due to a combination of precipitation with proteins and precipitation due to oxidative polymerization.[16]
- Mash duration - A longer mash duration extracts more phenolic content.[53][16]
- Stirring - The amount of extracted arabinoxylans and therefore the amount of bound phenolic acids (mainly ferulic acid) is increased by stirring the mash.[24]
- Oxidation - Oxygen exposure during mashing (see Oxidation) dramatically decreases the concentration of polyphenols during mashing.[52][53][5] Conversely, low oxygen brewing and the addition of antioxidants leads to the production of wort with increased phenolic content.
- Added enzymes - Exogenous cinnamoyl esterase enzymes can be used during mashing to increase the phenolic acid content in wort.[15]
- Sparge pH - High/uncontrolled pH during mashing or sparging will increase the extraction of undesirable harsh/astringent compounds, generally thought to be phenols.[5][55][27] Brewers (especially those that sparge) should control brewing pH, and in particular avoid alkalinity in the sparge water and excessively high temperature.
Ultimately, only a small portion of phenolic acids in malt are extracted into wort during mashing, the majority remains in the spent grain.[1][16] A substantial amount of bound phenolic acids (especially ferulic acid) are extracted, which may have effects later during fermentation or aging.[2] Varying amounts of polyphenols are extracted; for example, 100% of flavanol monomers are extracted but only 23% of polymers (4+ units).[1][27]
The mashing process is very complex and dynamic, consisting of a balance between the release of bound phenolic compounds, and their degradation.[16][56]
Hops[edit]
Hops are very high in phenolic content, containing around 4–14% total phenolics by dry weight.[16][1][44][2] The bulk of these phenolics are located within the hop cone plant material (the green part of the hop flower).[2][16] The amount of phenolic compounds depends on the hop variety, growing conditions, and harvest time.[16][1] For example, the polyphenol content of hops tends to be higher when they are harvested earlier.[1] The diverse array of hop phenolic compounds include many that differ from those found in grain.[1][16][57] Xanthohumol is one such compound notable for its significant health benefits and anticarinogenic properties, and brewers can take certain steps to increase the amount of this compound in the beer.[16][1][44] Hop prenylated flavonoids are extremely stable over the course of beer storage.[1]
Factors affecting phenolic compound extraction from hops:
- Cultivar - Among hop cultivars, the lower bitterness (low α-acid) varieties often contain higher levels of phenolic compounds.[44][12][58] Magnum hops are a notable exception, a high α-acid variety that also has been shown to contribute high polyphenol content.[12]
- Product form - Differing amounts of phenolics will be extracted into the wort based on the type of hop product used.[44][16] Fresh (wet) hops contain the highest levels of phenols, which can help improve flavor stability compared to other hop products. The drying and grinding process (i.e. turning fresh hops into pellets or plugs) causes losses in polyphenol content and antioxidant activity.[1][16][44] Hop pellets are available in 2 types: type-90 and type-45, which contain 90% and 45%, respectively, of raw hop cone plant material (which is where the phenolic compounds are located).[2] Therefore the type-90 pellets are much higher in phenolic content than type-45 pellets. Hop extracts are another hop product form, and the polyphenol content of these depends on the extraction solvent used during production.[1] CO2 hop extracts contain practically no phenolics.[44]
- Product age - The total polyphenol content and antioxidant activity of hops decline significantly during storage.[1][58]
- Stage of addition - Differing amounts of phenolics will be passed to the beer based on the stage of addition.[44] Approximately 50–70% of the total hop phenolics are extracted when added during wort boiling.[1] A similar percentage is extracted during dry hopping,[16] however, much more of the phenolics from this late stage hop addition are retained in the final beer (see below). It takes about 2–3 days for maximum phenolic compound extraction during dry hopping, although it may take more time at lower temperatures.[16]
- Hopping rates - Using more hops will increase the phenolic content of the beer (with other factors being equal).
- Oxidation - Oxidation that occurs after the hops are added will cause removal of some portion of the phenolic compounds that were extracted, especially during the stages of wort chilling and beer storage.
Fruit and other plants[edit]
Fruits tend to contain high levels of phenolic compounds, and therefore the use of fruit (or fruit juice) as a brewing ingredient will likely increase the total phenolic content and antioxidant activity of the beer.[45][59] Many fruits have been studied as brewing ingredients: carnelian cherry juice,[45][11] quince,[45][11][60] persimmon,[45][11] goji berries,[45][11] jackfruit,[45] green pepper,[11] and grapes.[45] Many non-fruit plants have also been studied and shown to increase phenolic content. Examples include: sedge,[45] diamond flower,[45] agarwood,[45] thyme,[11] juniper,[11] hibiscus,[11][61], green tea,[11] reishi mushroom,[11] chickling,[11] lentils,[11] sweet potato,[11] buckwheat,[11] cocoa,[8] walnut,[8] chestnut,[8] licorice,[8] coffee,[8] olive leaves,[62] and lemon balm.[11] These ingredients may also positively or negatively affect the other characteristics of the beer (flavor, color, clarity, pH value).[11][61] Cinnamon has modest antioxidant capacity[63][64] and has been used by home brewers during mashing for some perceived benefit without substantial flavor contribution.[65][66] Gluten-free beer made with white sorghum has shown to contain higher levels of phenolic compounds.[11] Blue corn used in brewing also provides high levels of phenolics.[11]
Honey (a plant derivative) contains phenolic compounds that can increase the phenolic content of beer.[8][67] Other honey bee products, such as propolis extract[45] and "bee pollen",[68] have also been shown to be useful for increasing phenolic content.
It's difficult to make recommendations regarding the use of any of these plants specifically for the purpose of increasing phenolic content of beer because they all may impact the flavor profile or other qualities of the beer for better or worse. Brewers may wish to experiment and judge the results for themselves, or simply use a tannin additive as a safer alternative.
Wood[edit]
Aging a beverage in contact with wood such as a barrel or on wood chips will impart it with phenolic compounds.[2] Higher toasted wood will generally contribute more phenolics. See Wood for more information regarding wood aging.
Additives[edit]
Brewers can add purified sources of phenolic compounds in order to gain the benefits of phenolic compounds without negatively affecting the flavor profile of the beer. Phenolic additives such as hop polyphenol extract and tannin extract have been successfully used to improve characteristcs of beer,[1][69][70][71][72][73] including:
- Improved flavor stability, improved malt and hop characteristics, and perceived freshness, especially when added during mashing
- Reduced bitterness, but prolonged stability of the iso-α-acids (including improved light stability)
- Improved foam stability
- Improved body
- Decreased color pickup
- Improved clarity, especially when combined with proper pH adjustment
- Improved lautering (when used in the mash)
- Decreased lipid oxidation and lipid carryover to the fermentation vessel (when used in the mash)
Because of these significant benefits, use of a tannin product during mashing is recommended as part of a low oxygen brewing process. It's important to note that these products are a preventative measure — they do not reverse oxidation reactions that have already occurred (including the formation of cardboard-like flavors during mashing).[69]
The most benefit is obtained from adding tannins at the beginning of mash-in. However, an extra dose can be added in the boil (before any other fining agents) in order to help with clarification. Tannins added to the boil can bind additional proteins that have been denatured by the heat and now have binding sites exposed that were unavailable during mashing. Tannins can be added even later (right before the final filtration before packaging),[74] however this may be impractical and of limited value.
See Tannin additives for product information.
Fate of phenolic compounds[edit]
Phenolic compounds are extracted into the wort and beer from the sources mentioned above, but the story doesn't end there. Many of the extracted phenolic compounds are removed or modified before the beer is consumed.[1][7] During mashing, boiling, and chilling, a great proportion of malt phenolics will be lost through precipitation or degradation.[44][1][75][16][12][7] Precipitated phenolics are removed along with the spent grains or the trub material. Fermentation and lagering result in further decreases in phenolic substances, since they are adsorbed to yeast cells or stabilizing agents, or precipitate due to oxidation.[1][7] Polyphenols are more easily removed than monophenolic compounds.[1][76] Keep in mind that removal of phenolic compounds is accompanied by a decrease in antioxidant activity and reduced flavor stability.[1][75]
Phenolic compound removal (at all stages):
- Oxidation during mashing, boiling, and chilling promotes polymerization, which leads to precipitation and removal with spent grains or trub.[1][27][38][71][77] Oxidative polymerization occurring during beer storage further decreases phenolic content and causes haze.[1][27][78][61][58] Oxidation not only causes precipitation, but also degrades some phenolics to other types of compounds, such as quinones.[44] Lower pH tends to increase polymerization.[12]
- Proteins bind and remove phenolic compounds.[16] Interestingly, less oxidized polyphenols tend to precipitate more readily with proteins.[1] Boiling for longer periods (over 60 minutes) causes increased elimination of phenolic compounds due to increased precipitation,[1][12] possibly due to increased binding with proteins.
- Stabilizing agents (such as PVPP) bind and remove phenolic compounds.[1]
- Yeast cells can bind phenolic compounds, although they may be released later.[1][79][16][7] Despite some removal of phenolic compounds at this stage, antioxidant capacity is generally unaffected by fermentation.[11][80]
Changes during boiling:
- A minor portion of cinnamic acid-derivatived phenolic acids are transformed by the heat to more flavor-active compounds during stages of high temperature, including kilning, boiling, whirlpool, and pasteurization.[1][4][44][81][56][16][82] Most importantly, ferulic acid is converted to 4-vinylguaiacol (4VG),[24] and when boiling for an extended period (e.g. 3 hours), the level of 4VG may approach sensory threshold concentration (0.3 mg/L).[2] (See phenolic acid derivatives below)
Changes during fermentation:
- Ferulic acid may increase during fermentation, which is attributed to feruloyl esterase activity in yeast liberating ferulic acid from dissolved arabinoxylans, especially from wheat.[1][56][16][44]
- Cinnamic acid-derivatived phenolic acids can be transformed by certain microbes to more flavor-active compounds. This typically occurs via phenylacrylic acid decarboxylase (Pad1-enzyme) and ferulic acid decarboxylase activity of top-fermenting "phenolic off-flavor positive" (POF+) Saccharomyces cerevisiae yeast strains.[24][2][81][16] In beers brewed with POF+ strains of S. cerevisiae, 4VG is the most prevalent volatile phenol, as ferulic acid dominates the wort cinnamic acid-derived phenolic acids and ferulic acid is preferentially degraded by yeast.[2][44] Some contaminating microorganisms like wild Saccharomyces spp., Brettanomyces spp., or Enterobacteriacea can have the same effect, either by Pad-1 activity or phenolic acid decarboxylase activity.[44][81][2] When POF+ yeast are used, transformation of ferulic acid occurs at a constant rate through fermentation and then decreases sharply during secondary fermentation.[44] The flavor-active vinyl compounds such as 4VG can be further oxidized or reduced into smaller molecules like vanillin, 4-ethylguaiacol, guaiacol, and 4-ethylphenol through chemical reactions or through the activity of wild microbes (especially by Brettanomyces).[44][82] See phenolic acid derivatives below for more information.
Changes during storage:
- Flavor-active monophenols (such as 4VG) transform during storage, changing the flavor (see phenolic acid derivatives below).[1]
Antioxidant activity and oxidation[edit]
Phenolic compounds (especially polyphenols) have long been considered the primary natural antioxidants in brewing raw materials and beer.[1][83][84][85][6][24][81][38][10][45][11] In other words, phenolics are the main defense against the oxidation of other sensitive substances (such as lipids).[83][5][15][44][86] It has been shown that malt, wort, and beer with higher total phenolic content display increased antioxidant activity, thus improving flavor stability.[83][35][87][88][85][6][35][89][69][90][91][79][49][75][9][33][70][12] The protective effect of phenols is particularly important during mashing,[83][44][70] where lipid concentration is high, oxidative enzymes are active, and oxygen exposure is difficult to avoid entirely. Due to their antioxidant activity, higher levels of phenolic compounds can help with faster lautering.[71] Phenolic antioxidant activity also helps to protect yeast viability against the stress generated by high levels of ethanol, enhancing the fermentation process and increasing attenuation.[32] Traditionally, the highest phenolic content in wort (and corresponding antioxidant activity) occurs after boiling due to the addition of hops,[76] although phenolic compounds may peak later depending on the amount of dry hopping. However, there are a large amount of insoluble phenolic compounds present in the grain, so the antioxidant effect of phenolic compounds may actually be higher during mashing.[38][92][93]
It's important to understand that phenolics help to protect the wort and beer only as long as oxygen exposure is minimized during the brewing process.[94][38][71][95] The antioxidant capacity can easily be overwhelmed, leading to rapid oxidation of other compounds as well as the emergence of various negative aspects of oxidized phenolics.[94] Therefore, it is beneficial to minimize oxygen exposure during brewing.
Some phenolic compounds provide greater antioxidant activity than others.[96] Generally the smaller phenolic compounds (e.g. flavonols, flavan-3-ols, and phenolic acids) are considered to be the most effective antioxidants in wort and beer.[1][37][89][20][44][87][9][86] However, ferulic acid bound to arabinoxylans is a more potent antioxidant than free ferulic acid.[97]
Phenols act as antioxidants in three ways:
- They neutralize reactive oxygen species (ROS)
- They act as chelating agents
- They inhibit lipoxygenase (LOX) enzymes
Oxygen scavenging[edit]
Polyphenols are the single most important group of compounds for absorbing oxygen introduced into wort and beer.[77][88][98] This is because phenolic compounds readily become oxidized by reactive oxygen species (ROS) in the wort/beer, effectively delaying the oxidation of other compounds.[6][76] The oxygen scavenging reactions can proceed by different mechanisms: free radical inactivation by hydrogen atom transfer (HAT), or single electron transfer (SET) reactions.[99][1][100][74] When phenolic compounds react with free radicals by HAT or SET, the reaction product includes a phenolic radical. Due to the aromatic ring structure, the phenolic radical electron is resonance-stabilized and therefore relatively non-reactive, thus effectively terminating the radical chain reaction.[45][1][6][96][100][86] Certain phenolic radicals (e.g. semiquinones resulting from flavan-3-ol oxidation) can also bind together (polymerize), forming non-radicals and thereby preserving their radical-scavenging capacity.[6][44] During mashing, phenolic compounds are also key substrates for oxidative enzymes including peroxidases, and thus enzymatically absorb oxygen after it is converted to hydrogen peroxide.[77][5][53] Through these mechanisms, polyphenols take up much more oxygen than any other compounds in wort/beer.[88][44] Oxidized phenolic compounds can be reduced through the action of other antioxidants such as sulfite or ascorbic acid.[101]
However, oxygen scavenging comes at a cost, particularly if oxygen exposure is not minimized during the brewing process. Here's a summary of the negative effects of phenolic compound oxidation:
- Oxidation of the ferulic acid bound to arabinoxylans causes cross-linking and precipitation during mashing or lautering, which may cause a stuck mash and also results in decreased body and lower dietary fiber content.[102]
- Malt polyphenols that become oxidized during brewing (on the hot side) are partly removed, and therefore are no longer available to help protect the beer from oxidation during packaging and storage.[5][53]
- Oxidized phenolic compounds give darker color.
- Oxidized phenolic compounds contribute increased astringency and harsh/bitter flavors.
- Oxidation causes phenolic compounds to link with proteins, forming haze.
- Oxidized polyphenols can slowly oxidize other substances (playing a pro-oxidative role).
Chelating activity[edit]
Even trace amounts of transition metals (especially iron and copper) catalyze the formation of ROS from O2, which promote rapid oxidation of various compounds. Polyphenols and phenolic acids strongly bind to these metals (mainly iron), forming stable complexes so that the metals do not readily initiate oxidative reactions.[44][1][20][6][74] Chelation is stronger at mashing pH compared to beer pH.[64]
Oxidative enzyme inhibition[edit]
Malt polyphenols are known to inhibit the action of lipoxygenase (LOX) enzymes, helping to prevent enzymatic lipid oxidation during mashing.[38][6][100][103][104][70][86][77] High concentrations of polyphenols can also inhibit polyphenol oxidase.[28]
Prooxidant activity[edit]
Phenolic compounds, when oxidized, can contribute to ROS formation and oxidative degradation of other compounds during beer aging (even when oxygen is no longer present).[26][88][5][85] This will typically occur relatively slowly due to the fairly low reactivity of oxidized phenolic compounds (i.e. radicals and quinones). Despite the overall protective effect of phenols, certain phenolic compounds (in particular the trihydroxyflavans, also called prodelphinidins) are especially prone to acting as oxidizing agents, reducing flavor stability.[55][83][87][20][36]
Prooxidant activity can also occur by another mechanism. Many substances with antioxidant activity might exert prooxidant activity when combined with transition metal ions. Phenolic compounds reduce these metal ions, which then catalyze the formation of ROS by Fenton and Haber-Weiss reactions (a process called redox cycling).[1][6][44][105] This effect is concentration-dependent — phenolic compounds act as prooxidants when the concentration of metals is relatively high, which may be relevant when using inadequately passivated stainless steel or other system components made of copper or aluminum such as wort chillers or sparging apparatus, or using brewing water with high content of metal ions. Ascorbic acid is another substance that can sometimes act as a prooxidant in this manner.[105]
Effect on color[edit]

In addition to the influence of malt on beer color, there is a strong effect of oxidation and phenolic content. As oxygen exposure increases during the brewing process and/or beer storage, phenolic substances become oxidized and polymerize, resulting in darker colored wort and beer.[1][27][5][44][88][52][106][107][26][108] In particular, the smaller flavanols are precursors for oxidative color formation. The "browning" effect can occur through various oxidation mechanisms, including autoxidation (direct reaction with reactive oxygen species), metal ion-catalyzed oxidation, or enzymatic reactions.[28][109][52] Beer styles with higher levels of phenolic compounds such as IPAs have a greater potential to darken since they contain more of the phenolic precursors that can become brown when oxidized.[32][110] It's important to note that higher levels of phenolic compounds do not necessarily affect beer color — it's only when they oxidize that the color changes.[70] Brewers can produce lighter colored wort and beer by minimizing oxidation throughout the brewing, packaging, and storage stages.[111][112]
| Phenolic Compound | Color | Color When Oxidized |
|---|---|---|
| Ferulic acid | White | Reddish brown |
| p-Coumaric acid | Yellow | Pale brown |
| Vanillic acid | White | Pale brown |
| 4-Hydroxybenzoic acid | White | Pale brown |
| (+)-Catechin | Cream | Reddish brown |
| Quercetin | Yellow | Pale brown |
| Kaempferol | Yellow | Coffee brown |
Effect on flavor and mouthfeel[edit]
Phenolic compounds can indirectly affect flavor through their action as antioxidants (see Oxidation and Flavor stability), but they can also have a direct impact on flavor and mouthfeel. The direct effect on flavor occurs through two different mechanisms. First, the phenolics naturally present in beer can cause unwanted harsh flavors and astringency when they become oxidized — a widespread problem since oxidation is often not properly avoided. Second, the phenolic acids can be modified during the brewing or fermentation steps in order to produce a wide variety of "phenolic" flavors.[27] This second mechanism usually only occurs in specialty beers where particular brewing strains or sometimes wild yeasts are used for fermentation in order to create flavorful compounds using phenolic acids as precursors.[2] Phenolic acids are also modified by heat, potentially resulting in phenolic flavors under certain conditions. Finally, chlorine compounds in tap water or residual halogen-based (e.g. chlorine or iodine) sanitizing agents can modify phenolics to create nasty off-flavors even at very low levels (chlorophenols; see chlorine removal).[27][55][113]
It is generally recognized that polyphenols contribute to the overall mouthfeel of beer, in particular to fullness.[70] This aspect of phenolic compounds is not well-studied in beer.
One interesting note is that gallic acid (a phenolic acid) is known to induce a distinct and long-lasting sweet aftertaste, providing a pleasant tasting experience.[114] Some studies show that gallic acid is the predominant phenolic acid in beer, whereas other studies show it to be entirely absent. These polarized results could mean that gallic acid is rather fragile (susceptible to destruction by oxidation),[115] and its presence may be part of the reason why unoxidized beer tastes so fresh and pleasant.
Bitterness, harshness, sourness, and astringency[edit]
Phenolic compounds (especially flavonol glycosides, flavanols, and phenolic acid ethyl esters) bind to proteins in the mouth, imparting an astringent sensation and flavors of bitterness, harshness, bitter-sweetness, and sourness.[1][94][5][27][6][88][83] Oxidized polyphenols in beer generally have lower flavor thresholds as compared to their precursors, making them more potent.[88] In particular, harshness, bitterness, and astringency are generally more pronounced in oxidized samples, and oxidation may produce other undesirable off-flavors as well, contributing to aged character (see table below).[88][55][27][44][113][93] On the other hand, non-oxidized (reduced) polyphenols are associated with beer freshness.[88] In malt-forward beers, the term herbstoffe is sometimes used to describe the harsh grain bitterness present when phenolic compounds become oxidized, which is a different flavor than hop α-acid bitterness.[55][113] Heavily dry hopped beers can cause a more extreme form of this sensation, commonly referred to as hop burn.[116] It is characterized by a harsh astringency upon swallowing the beer, due to the high amount of phenolic compounds in suspended particles,[16] and it is frequently exacerbated by dry hopping methods that promote oxidation. The occurrence of all these off-flavors from phenolic compounds is avoidable by holistic brewing practices designed to minimize oxidation.
While it's clear that off-flavors and astringency resulting from oxidized phenolic compounds are problematic and are very likely responsible for the "harsh" notes that many conventional high-oxygen brewers detect in their young beer, not all hope is lost for those beers. Oxidation increases the polymerization of certain polyphenols, and the resulting large compounds will eventually precipitate.[44][1] Consequently, the harsh flavors resulting from oxidation generally tend to improve while aging over a period of days to months, giving the offending compounds time to precipitate and settle or degrade.[83][116][1][21] However, aging the beer to remove these off-flavors is not ideal, especially since other types of flavor deterioration will occur during that time. Therefore, low oxygen brewing can be a solution for brewers looking to produce beer without this harsh, bitter, astringent character, and/or produce beer that is drinkable much more quickly.
| Major flavors | Minor flavors | |
|---|---|---|
|
|
|
* decreases when oxidized
† increases when oxidized
Phenolic flavors (volatile phenols)[edit]
Phenolic acids (such as ferulic acid) are always present in beer, but they typically have no direct flavor impact since their concentrations are well below flavor threshold.[1][24][2][27][44] However, they act as flavor precursors because they can be modified during brewing and/or fermentation to create much more potent flavor compounds, called volatile phenols.[1][24][2][82][97] The aroma and flavor profile contributed by volatile phenols in a beer is influenced by not just by the concentrations of individual phenolic compounds, but also the relative amounts, the total concentration of phenolics, their interactions with other compounds, and variation among individuals for sensitivity to these compounds.[2] The resulting flavors may be desirable, or they may be considered off-flavors depending on the beer style. Styles that intentionally feature volatile phenolic flavors include weissbier, rauchbier, saison, and various Belgian beers such as witbier, tripel, and traditional sour beers.[24][2][27] In these styles, volatile phenols often impart pleasant spicy, clove-like, sweet, and vanilla-like flavor notes, although, at higher concentrations they may be also add unpleasant medicinal flavor notes.[1][2][27][58] The "black pepper" character often present in certain styles is considered a phenolic flavor, however the compounds responsible have not been identified.[2] Depending on the yeast(s), other volatile phenols can be produced that contribute "funky" flavors such as barnyard, horsey, leathery, and smoky.[2] Brettanomyces yeast is notable for creating these funky flavors.[58] Since volatile phenols are mainly formed during fermentation, the choice of an appropriate yeast strain is the primary way to control the final volatile phenol character in beer.[2][24][27] Among yeast varieties, the so-called "phenolic off-flavor"-positive (POF+) strains are responsible for creating the volatile phenols.[2][56] A "ferulic acid rest" during mashing may be useful to increase the level of the precursor if a high clove flavor (4-vinylguaiacol) level is desired.[2] Apart from the desired phenolic aroma-active substances, the toxic compound styrene is formed via the same pathway.[1][4]
Phenolic flavors can become undesirable if the level becomes too high, or even if the character could be considered pleasant (spice, clove). This could occur due to inappropriate yeast strain choice (inadvertently choosing a POF+ strain instead of a typical brewing strain), or fermentation at a temperature above the recommended range.[2] In some cases, strong and offensive medicinal, plastic strip (Band-Aid®), goaty, burnt, or creosote (tar) character can be present, among others.[27] For beers that are dominated by offensive or unexpected phenolic character, the most likely source of volatile phenols is contaminating spoilage organisms.[2]
Aside from microbial/fermentation issues, phenolic compounds are transformed during beer storage/aging and the products are potential contributors to stale flavor — even in beers that did not start with significant phenolic flavors.[44][2] In particular, volatile phenols (4-vinylsyringol) may be at least partly responsible for the characteristic "old beer" aroma.[58][117] Lastly, beers with a desirable clove/spice (4-vinylguaiacol) flavor may lose this character over time in favor of a sweeter taste (vanillin and/or apocynol).[2][81][27][58] This degradation occurs faster with higher storage temperature, with the presence of oxygen, and at lower pH (under 4.6).[81][85][1]
| Volatile phenol | Flavor description | Notes |
|---|---|---|
| 4-vinylguaiacol (4VG) | Low level: pleasant clove/spice flavor High level: pharmaceutical off-flavor |
Formed during boiling or fermentation. Derivative of ferulic acid. |
| 4-vinylphenol | Phenolic, medicinal, bitter | Always considered to be an off-flavor. Derivative of p-coumaric acid. |
| Guaiacol | Smoky | Formed during beer aging by degradation of 4VG. Major contributor to the "smoke" character in smoked malt. |
| 4-vinylsyringol | "Old-beer-like" | Very low taste threshold. It is released during aging. Derivative of sinapic acid. |
| Vanillin | Sweet, vanilla | Oxidation product of 4VG, which may change the flavor profiles of phenolic beers during aging. |
| 4-ethylguaiacol | Clove, phenolic, spice, woody, smoky, vanilla | Degradation product of 4VG during beer aging or yeast metabolism. |
Besides beer, volatile phenols have been reported to contribute to the aroma of wine, sherry, and whisky as well as nonalcoholic beverages such as fruit juices and coffee.[24][81] In wine, both vinylphenols (4VP and 4VG) and their ethyl analogues (4-ethylphenol and 4-ethylguaiacol) have been detected and are important contributors to aroma.[81]
Effect on haze[edit]
Phenolic compounds are directly involved in the formation of beer haze, typically by binding to proteins as a result of oxidation. See Haze for more information.
Effect on foam[edit]
Some studies have found a correlation between total phenolic content and foam stability in beer.[1][118][71] However, it appears that this is not a direct result of these compounds creating or stabilizing foam, but rather some other mechanism.[83][69][91][70] One possibility is that phenolic compounds help indirectly by preventing oxidation of lipids (oxidized lipids are potent foam-negative compounds). See Foam for more information.
Effect on human health[edit]
Phenolic compounds show a wide spectrum of beneficial health-related properties. See Health and safety.
See also[edit]
References[edit]
Note: When looking at studies, keep in mind that the Folin-Ciocalteu method measures constituents other than phenolics, and its specificity is poor.[51][119][13][21]
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo Wannenmacher J, Gastl M, Becker T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr Rev Food Sci Food Saf. 2018;17(4):953–988.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae Lentz M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation. 2018;4(1):20.
- ↑ a b Dabina-Bicka I, Karklina D, Kruma Z. Polyphenols and Vitamin E as potential antioxidants in barley and malt. Conference Proceedings of the 6th Baltic Conference on Food Science and Technology FOODBALT-2011; May 5–6, 2011; Jelgava, Latvia.
- ↑ a b c d e f Schwarz KJ, Boitz LI, Methner FJ. Release of phenolic acids and amino acids during mashing dependent on temperature, pH, time, and raw materials. J Am Soc Brew Chem. 2012;70(4):290–295.
- ↑ a b c d e f g h i j k Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b c d e f g h i j k l Aron PM, Shellhammer TH. A discussion of polyphenols in beer physical and flavor stability. J Inst Brew. 2010;116(4):369–380.
- ↑ a b c d e f Fumi MD, Galli R, Lambri M, Donadini G, De Faveri DM. Effect of full-scale brewing process on polyphenols in Italian all-malt and maize adjunct lager beers. J Food Compos Anal. 2011;24(4–5):568–573.
- ↑ a b c d e f g Nardini M, Foddai MS. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules. 2020;25(11):2466.
- ↑ a b c d Zhao H, Chen W, Lu J, Zhao M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010;119(3):1150–1158.
- ↑ a b Zhao H, Li H, Sun G, Yang B, Zhao M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J Sci Food Agric. 2013;93(4):910-917.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z Yang D, Gao X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci Technol. 2021;110:754-764.
- ↑ a b c d e f g h i Šibalić D, Planinić M, Jurić A, Bucić-Kojić A, Tišma M. Analysis of phenolic compounds in beer: from raw materials to the final product. Chem Zvesti. 2021;75(1):67–76.
- ↑ a b Ganbaatar C, Kubáň V, Kráčmar S, Valášek P, Fišera M, Hoza I. Liquid chromatographic determination of polyphenenols in Czech beers during brewing proces. Potravinárstvo. 2015;9(1):24–30.
- ↑ Niño-Medina G, Romo-Longoria JD, Ramírez-González IV, Martínez-Reyna OO, Urías-Orona V. Phenolic content and antioxidant capacity level in commercial Mexican lager beers. J Am Soc Brew Chem. 2017;75(2):156–158.
- ↑ a b c d e f g h i j k l Zhao H. Chapter 64: Effects of processing stages on the profile of phenolic compounds in beer. In: Preedy V, ed. Processing and Impact on Active Components in Food. Academic Press; 2015:533-539.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Carvalho DO, Guido LF. A review on the fate of phenolic compounds during malting and brewing: technological strategies and beer styles. Food Chem. 2022;372:131093.
- ↑ Piazzon A, Forte M, Nardini M. Characterization of phenolics content and antioxidant activity of different beer types. J Agric Food Chem. 2010;58(19):10677–10683.
- ↑ Floridi S, Montanari L, Marconi O, Fantozzi P. Determination of free phenolic acids in wort and beer by coulometric array detection. J Agric Food Chem. 2003;51(6):1548–1554.
- ↑ a b Carvalho DO, Gonçalves LM, Guido LF. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr Rev Food Sci Food Saf. 2016;15(5):927–943.
- ↑ a b c d e f Siqueira PB, Bolini H, Macedo GA. O processo de fabricação da cerveja e seus efeitos na presença de polifenóis. (The beer manufacturing process and its effects on the presence of polyphenols.) Alimentos e nutrição. 2008;19(4):491–498.
- ↑ a b c Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1):223S–229S.
- ↑ a b c Gerhäuser C, Becker H. Chapter 12: Phenolic compounds in beer. In: Preedy VR, ed. Beer in Health and Disease Prevention. Academic Press; 2009:124–144.
- ↑ Pihlava JM, Kurtelius T, Hurme T. Total hordatine content in different types of beers. J Inst Brew. 2016;122(2):212–217.
- ↑ a b c d e f g h i j k l m n o p Vanbeneden N, Van Roey T, Willems F, Delvaux F, Delvaux FR. Release of phenolic flavor precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008;111(1):83–91.
- ↑ Nardini M, Ghiselli A. Determination of free and bound phenolic acids in beer. Food Chem. 2004;84(1):137–143.
- ↑ a b c Savel J. Negative role of oxidised polyphenols and reductones in beer. BrewingScience - Monatsschrift Brauwiss. 2006;59(1/2):33–40.
- ↑ a b c d e f g h i j k l m n o p q r s t u v Habschied K, Košir IJ, Krstanović V, Kumrić G, Mastanjević K. Beer polyphenols—bitterness, astringency, and off-flavors. Beverages. 2021;7(2):38.
- ↑ a b c Quinde-Axtell Z, Baik BK. Phenolic compounds of barley grain and their implication in food product discoloration. J Agric Food Chem. 2006;54(26):9978–9984.
- ↑ Callemien D, Collin S. Use of RP-HPLC-ESI (–)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J Am Soc Brew Chem. 2008;66(2):109–115.
- ↑ Quifer-Rada P, Vallverdú-Queralt A, Martínez-Huélamo M, Chiva-Blanch G, Jáuregui O, Estruch R, Lamuela-Raventós R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food chem. 2015;169:336–343.
- ↑ Cheiran KP, Raimundo VP, Manfroi V, Anzanello MJ, Kahmann A, Rodrigues E, Frazzon J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food chem. 2019;286:113–122.
- ↑ a b c d Boronat A, Soldevila-Domenech N, Rodríguez-Morató J, Martínez-Huélamo M, Lamuela-Raventós RM, de la Torre R. Beer phenolic composition of simple phenols, prenylated flavonoids and alkylresorcinols. Molecules. 2020;25(11):2582.
- ↑ a b c d e Zhou B, Zhao J, Schwarz PB, Li Y. Effect of grinding and extraction conditions on the determination of antioxidant activity and phenolic acids in barley. J Food Meas Charact. 2021;15:3823–3836.
- ↑ Nordkvist E, Salomonsson AC, Åman P. Distribution of insoluble bound phenolic acids in barley grain. J Sci Food Agric. 1984;35(6):657–661.
- ↑ a b c d e f g Zhao H, Zhao M. Effects of mashing on total phenolic contents and antioxidant activities of malts and worts. Int J Food Sci Technol. 2012;47(2):240-247.
- ↑ a b Whittle N, Eldridge H, Bartley J, Organ G. Identification of the polyphenols in barley and beer by HPLC/MS and HPLC/electrochemical detection. J Inst Brew. 1999;105(2):89–99.
- ↑ a b Humia BV, Santos KS, Barbosa AM, Sawata M, Mendonça MdC, Padilha FF. Beer molecules and its sensory and biological properties: A review. Molecules. 2019;24(8):1568.
- ↑ a b c d e f Guido LF, Curto AF, Boivin P, Benismail N, Gonçalves CR, Barros AA. Correlation of malt quality parameters and beer flavor stability: multivariate analysis. J Agric Food Chem. 2007;55(3):728–733.
- ↑ Leitao C, Marchioni E, Bergaentzlé M, et al. Fate of polyphenols and antioxidant activity of barley throughout malting and brewing. J Cereal Sci. 2012;55(3):318–322.
- ↑ a b Dvořáková M, Guido LF, Dostálek P, Skulilová Z, Moreira MM, Barros AA. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J Inst Brew. 2008;114(1):27–33.
- ↑ Šimić G, Horvat D, Dvojković K, et al. Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J Food Sci. 2017;35(1):73–78.
- ↑ Dvořáková M, Douanier M, Jurková M, Kellner V, Dostálek P. Comparison of antioxidant activity of barley (Hordeum vulgare L.) and malt extracts with the content of free phenolic compounds measured by high performance liquid chromatography coupled with CoulArray detector. J Inst Brew. 2008;114(2):150–159.
- ↑ Koren D, Kun S, Vecseri BH, Kun-Farkas G. Study of antioxidant activity during the malting and brewing process. J Food Sci Technol. 2019;56(8):3801–3809.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Callemien D, Collin S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—a review. Food Rev Int. 2009;26(1):1–84.
- ↑ a b c d e f g h i j k l m n Martinez-Gomez A, Caballero I, Blanco CA. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules. 2020;10(3):400.
- ↑ Szwajgier D. Content of individual phenolic acids in worts and beers and their possible contribution to the antiradical activity of beer. J Inst Brew. 2009;115(3):243–252.
- ↑ Socha R, Pająk P, Fortuna T, Buksa K. Antioxidant activity and the most abundant phenolics in commercial dark beers. International journal of food properties. Int J Food Prop. 2017;20(sup1):1–15.
- ↑ Zhou B, Jin Z, Schwarz P, Li Y. Impact of genotype, environment, and malting conditions on the antioxidant activity and phenolic content in US malting barley. Fermentation. 2020;6(2):48.
- ↑ a b c Shopska V, Denkova-Kostova R, Dzhivoderova-Zarcheva M, Teneva D, Denev P, Kostov G. Comparative study on phenolic content and antioxidant activity of different malt types. Antioxidants. 2021;10(7):1124.
- ↑ Fogarasi A-L, Kun S, Tankó G, Stefanovits-Bányai É, Hegyesné-Vecseri B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015;167(15):1–6.
- ↑ a b c Zieliński H, Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48(6):2008–2016.
- ↑ a b c d Chapon L, Chemardin M. The dissolving and oxidation of malt tannoids on mashing-in. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1964;22(1):244–258.
- ↑ a b c d e f Narziss L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
- ↑ Andersen ML, Skibsted LH. Modification of the levels of polyphenols in wort and beer by addition of hexamethylenetetramine or sulfite during mashing. J Agric Food Chem. 2001;49(11):5232–5237.
- ↑ a b c d e Fix G. Principles of Brewing Science. 2nd ed. Brewers Publications; 1999.
- ↑ a b c d Langos D, Granvogl M. Studies on the simultaneous formation of aroma-active and toxicologically relevant vinyl aromatics from free phenolic acids during wheat beer brewing. J Agric Food Chem. 2016;64(11):2325–2332.
- ↑ Kellner V, Jurková M, Čulík J, Horák T, Čejka P. Some phenolic compounds in Czech hops and beer of Pilsner type. Brew Sci. 2007;60:31–37.
- ↑ a b c d e f g Collin S, Jerković V, Bröhan M, Callemien D. Polyphenols and beer quality. In: Ramawat KG, Mérillon J-M, eds. Natural Products. 1st ed. Springer; 2013:2334–2353.
- ↑ Baigts-Allende DK, Perez-Alva A, Ramirez-Rodrigues MA, Palacios A, Ramirez-Rodrigues MM. A comparative study of polyphenolic and amino acid profiles of commercial fruit beers. J Food Compos Anal. 2021;100:103921.
- ↑ Zapata PJ, Martínez-Esplá A, Gironés-Vilaplana A, Santos-Lax D, Noguera-Artiaga L, Carbonell-Barrachina ÁA. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. Lwt. 2019;103:139–146.
- ↑ a b c Martínez A, Vegara S, Herranz‐López M, et al. Kinetic changes of polyphenols, anthocyanins and antioxidant capacity in forced aged hibiscus ale beer. J Inst Brew.] 2017;123(1):58–65.
- ↑ Guglielmotti M, Passaghe P, Buiatti S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J Food Sci. 2020;85(8):2278–2285.
- ↑ Wijewardhana US. Gunathilaka UGSA. Navaratne SB. Determination of total phenolic content, radical scavenging activity and total antioxidant capacity of cinnamon bark, black cumin seeds and garlic. Int J Adv Eng Res Sci. 2019;4(2):55–57.
- ↑ a b https://onlinelibrary.wiley.com/doi/full/10.1002/jib.673
- ↑ Using cinnamon in the mash. American Homebrewers Association online forum. 2011. Accessed Jan 2023.
- ↑ Cinnamon in the mash. Homebrew Talk online forum. 2009–2012. Accessed Jan 2023.
- ↑ Annapoorani A, Anilakumar KR, Khanum F, Murthy NA, Bawa AS. Studies on the physicochemical characteristics of heated honey, honey mixed with ghee and their food consumption pattern by rats. Ayu. 2010;31(2):141–146.
- ↑ Solgajová M, Ivanišová E, Nôžková J, Frančáková H, Tóth Ž, Dráb Š. Antioxidant activity and polyphenol content of malt beverages enriched with bee pollen. J Microbiol Biotech Food Sci. 2014;3(3):281–284.
- ↑ a b c d De Francesco G, Bravi E, Sanarica E, Marconi O, Cappelletti F, Perretti G. Effect of addition of different phenolic-rich extracts on beer flavour stability. Foods. 2020;9(11):1638.
- ↑ a b c d e f g Jaskula-Goiris B, Goiris K, Syryn E, et al. The use of hop polyphenols during brewing to improve flavor quality and stability of pilsner beer. J Am Soc Brew Chem. 2014;72(3):175–183.
- ↑ a b c d e Karabín M, Hanko V, Nešpor J, Jelínek L, Dostálek P. Hop tannin extract: a promising tool for acceleration of lautering. J Inst Brew. 2018;124(4):374–380.
- ↑ http://www.themodernbrewhouse.com/wp-content/uploads/2017/02/Officiele_tekst_voor_Brewing_Science.pdf
- ↑ Ryder DS. Chapter 10 Processing aids in brewing. In: Stewart GG, Russell I, Anstruther A, eds. Handbook of Brewing. 3rd ed. CRC Press; 2017.
- ↑ a b c Lugasi A. Polyphenol content and antioxidant properties of beer. Acta Aliment. 2003;32(2):181–192.
- ↑ a b c Gorjanović SŽ, Novaković MM, Potkonjak NI, LeskoŠek-Čukalović I, Sužnjević DŽ. Application of a novel antioxidative assay in beer analysis and brewing process monitoring. J Agric Food Chem. 2010;58(2):744–751.
- ↑ a b c Szwajgier D. Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J Inst Brew. 2011;117(4):569–577.
- ↑ a b c d Stephenson WH, Biawa JP, Miracle RE, Bamforth CW. Laboratory-scale studies of the impact of oxygen on mashing. J Inst Brew. 2003;109(3):273–283.
- ↑ Siqueira PB, Bolini HMA, Macedo GA. Polyphenols and antioxidant properties in forced and naturally aged Brazilian beer. J Brew Distilling. 2011;2(3):45–50.
- ↑ a b Pascoe HM, Ames JM, Chandra S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J Am Soc Brew Chem. 2003;61(4):203–209.
- ↑ Leitao C, Marchioni E, Bergaentzlé M, et al. Effects of processing steps on the phenolic content and antioxidant activity of beer. J Agric Food Chem. 2011;59(4):1249–1255.
- ↑ a b c d e f g h Vanbeneden N, Saison D, Delvaux F, Delvaux FR. Decrease of 4-vinylguaiacol during beer aging and formation of apocynol and vanillin in beer. J Agric Food Chem. 2008;56(24):11983–11988.
- ↑ a b c Vanbeneden N, Delvaux F, Delvaux FR. Determination of hydroxycinnamic acids and volatile phenols in wort and beer by isocratic high-performance liquid chromatography using electrochemical detection. J Chromatogr A. 2006;1136(2):237–242.
- ↑ a b c d e f g h Mikyška A, Hrabak M, Hašková D, Šrogl J. The role of malt and hop polyphenols in beer quality, flavour and haze stability. J Inst Brew. 2002;108(1):78–85.
- ↑ Guido LF, Boivin P, Benismail N, Gonçalves CR, Barros AA. An early development of the nonenal potential in the malting process. Eur Food Res Technol. 2005;220:200–206.
- ↑ a b c d Walters MT, Heasman AP, Hughes PS. Comparison of (+)–catechin and ferulic acid as natural antioxidants and their impact on beer flavor stability. Part 2: Extended storage trials. J Am Soc Brew Chem. 1997;55(3):91–98.
- ↑ a b c d Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79(12):1625–1634.
- ↑ a b c Irwin AJ, Barker RL, Pipasts P. The role of copper, oxygen, and polyphenols in beer flavor instability. J Am Soc Brew Chem. 1991;49(3):140–149.
- ↑ a b c d e f g h i j k Dadic M, Belleau G. Polyphenols and beer flavor. Proceedings of the Annual meeting of the American Society of Brewing Chemists. 1973;31(1):107–114.
- ↑ a b Jurková M, Horák T, Hašková D, Čulík J, Čejka P, Kellner V. Control of antioxidant beer activity by the mashing process. J Inst Brew. 2012;118(2):230-235.
- ↑ Fantozzi P, Montanari L, Mancini F, et al. In vitro antioxidant capacity from wort to beer. LWT - Food Sci Technol. 1998;31(3):221–227.
- ↑ a b Habschied K, Lončarić A, Mastanjević K. Screening of polyphenols and antioxidative activity in industrial beers. Foods. 2020;9(2):238.
- ↑ Maillard M-N, Berset C. Evolution of antioxidant activity during kilning: role of insoluble bound phenolic acids of barley and malt. J Agric Food Chem. 1995;43(7):1789–1793.
- ↑ a b McMurrough I, Madigan D, Kelly RJ, Smyth MR. The role of flavanoid polyphenols in beer stability. J Am Soc Brew Chem. 1996;54(3):141–148.
- ↑ a b c Kunze W. Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019.
- ↑ Frederiksen AM, Festersen RM, Andersen ML. Oxidative reactions during early stages of beer brewing studied by electron spin resonance and spin trapping. J Agric Food Chem. 2008;56(18):8514–8520.
- ↑ a b Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: structure-activity relationship. Biosci Biotechnol Biochem. 1992;56(2):324–325.
- ↑ a b Szwajgier D, Pielecki J, Targoński Z. The release of ferulic acid and feruloylated oligosaccharides during wort and beer production. J Inst Brew. 2005;111(4):372–379.
- ↑ Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ Leopoldini M, Marino T, Russo N, Toscano M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A. 2004;108(22):4916–4922.
- ↑ a b c Maillard M-N, Soum M-H, Boivin P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. LWT - Food Sci Technol. 1996;29(3):238–244.
- ↑ Nardini M, Cirillo E, Natella F, Mencarelli D, Comisso A, Scaccini C. Detection of bound phenolic acids: prevention by ascorbic acid and ethylenediaminetetraacetic acid of degradation of phenolic acids during alkaline hydrolysis. Food Chem. 2002;79(1):119–124.
- ↑ Egi A, Speers RA, Schwarz PB. Arabinoxylans and their behavior during malting and brewing. Tech Q Master Brew Assoc Am. 2004;41(3):248–267.
- ↑ Cos P, Bruyne TD, Hermans N, Apers S, Berghe DV, Vlietinck AJ. Proanthocyanidins in health care: Current and new trends. Curr Med Chem. 2004;11(10):1345–1359.
- ↑ Hoult JR, Moroney MA, Payá M. Chapter 44: Actions of flavonoids and coumarins on lipoxygenase and cyclooxygenase. In: Colowick SP, Abelson JN, eds. Methods in Enzymology. Vol 234. Academic Press; 1994:443–454.
- ↑ a b Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic Biol Med. 1997;22(5):749–760.
- ↑ Hodžić Z, Karahmetović A, Saletović M, Šestan A. Relationship between total polyphenols and colour values of wort and beer. J Eng. 2007;1:1584–2665.
- ↑ Clarkson SP, Large SJ, Bamforth CW. Oxygen-scavenging enzymes in barley and malt and their effects during mashing. J Inst Brew. 1992;98(2):111–115.
- ↑ Kunz T, Brandt NO, Seewald T, Methner FJ. Carbohydrates addition during brewing – effects on oxidative processes and formation of specific ageing compounds. BrewingScience. 2015;68(7):78–92.
- ↑ Quinde-Axtell Z, Powers J. Baik BK. Retardation of discoloration in barley flour gel and dough. Cereal Chem. 2006;83(4):385–390.
- ↑ Chris. How to reduce oxidation of New England hazy IPAs. Beer Maverick website. July 2022. Accessed May 2023.
- ↑ Biering J. Reliable scale up/scale down in process development—New possibilities to close the gap between lab, pilot brewery, and industrial scale. Slides presented at: Annual meeting of American Society of Brewing Chemists. June 4–7, 2017; Fort Myers, FL.
- ↑ Rabe B. What does oxidation look like? The Modern Brewhouse website. 2016. Accessed online March 2024.
- ↑ a b c Prechtl C. Some practical observations concerning grain bitterness in beers and its amelioration. Tech Q Master Brew Assoc Am. 1967;4(1):98–103.
- ↑ https://patents.google.com/patent/US20020068123A1/en
- ↑ Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50(7):2161–2168.
- ↑ a b Naglich M. How to avoid ‘hop burn’ when homebrewing hazy IPAs. VinePair website. 2020. Accessed May 6, 2022.
- ↑ Callemien D, Dasnoy S, Collin S. Identification of a stale-beer-like odorant in extracts of naturally aged beer. J Agric Food Chem. 2006;54(4):1409–1413.
- ↑ Jurić A, Ćorić N, Odak A, Herceg Z, Tišma M. Analysis of total polyphenols, bitterness and haze in pale and dark lager beers produced under different mashing and boiling conditions. J Inst Brew. 2015;121(4):541–547.
- ↑ Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18(2):2328–2375.