Beta-glucans and arabinoxylans
Classified as non-starch polysaccharide (NSP) carbohydrates, β-glucans and arabinoxylans are major structural components of the cell walls in cereal grain, mainly located within the barley endosperm and husk.[1][2][3] NSPs are important because they can have direct effects on various processes in the brewery and on the quality of the final beer.[4] Undegraded NSPs can cause negative effects such as haze, lower extraction, increased wort viscosity, filtering difficulties, and gel formation, causing a stuck mash. The scientific brewing literature generally paints NSPs as problematic compounds, but really their negative effects are largely irrelevant or avoidable, particularly on the home brew scale. Home brewers generally do not filter the beer, nor do they suffer from slow lautering. Stuck mash issues are solved by minimizing oxygen during the mashing process along with proper milling. On the other hand, NSPs can offer benefits such as providing a more full mouthfeel, increased foam stability, and dietary benefits.
NSPs are partially extracted into the wort during mashing. The size of the NSPs extracted is of more importance than the quantity, and both aspects mainly depend on the degree of malt modification rather than any mashing parameters.[5][3][6][7][8] Larger (high molecular weight) molecules are mostly responsible for the effects these molecules cause. Lower malt modification increases the amount of large NSPs extracted — poorly modified malt can increase the wort β-glucan content by up to 14 times as much as that from a well-modified malt, along with up to a two-fold increase in large arabinoxylans.[6][5][9] Similarly, unmalted barley delivers increased amounts of large β-glucans into the wort.[10][11][12] An extended step mashing rest at 113–119°F (45–48°C) can degrade some of the NSPs to help avoid problems associated with excessive levels.[7] However, this low temperature rest is unnecessary for home brewers in most cases, and it can be detrimental to beer quality overall because enzyme activity at this temperature tends to result in poor head retention, reduced beer body, and lower flavor stability.[7]
Molecular structure and content in grain[edit]
β-glucans and arabinoxylans are categorized as non-starch polysaccharides because they are made up of long chains of sugars, but with different linkages and/or different sugars than starch. β-glucan is a linear (non-branched) polymer of glucose units bound together by a mixture of (1,4) and (1,3)-β-glycosyl linkages.[1][13][7] β-glucans do not have other molecular groups attached to them.[13]
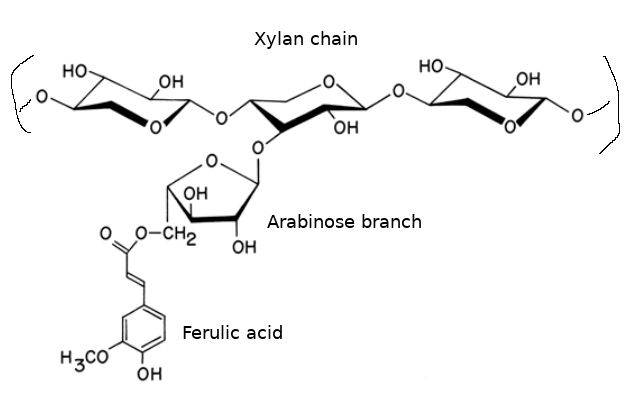
Arabinoxylans (a subset of pentosans) are polymers of the 5-carbon sugar xylose, bound together by (1,4)-β-xylopyranosyl linkages.[3][1][6][2] The chain has side groups attached to some of the xylose units, especially arabinose (another 5-carbon sugar), which is bound with a (1,3)-β link.[3][6][14] Various other small molecules are attached to the chain as well, including galactose, glucuronic acid, methyl, acetyl, and ferulic acid groups.[1][14] Ferulic acid (present in barley, wheat, and rye) is bound to arabinose via an ester bond.[6][2][9] Arabinoxylans can crosslink with proteins and other arabinoxylans (via the oxidative connection of two ferulic acid molecules or via the amino acid tyrosine).[6][9]
| Grain | β-glucan | Arabinoxylan |
|---|---|---|
| Barley | 3–5%[3][7][2] | 5–11%[3][9] |
| Oats | 3–7%[2] | 3–6%[9] |
| Wheat | < 0.5%[2][15][16] | 6–13%[15][9] |
| Rye | 2%[2] | 8–10%[9] |
| Maize | < 0.5%[15] | 2–3%[15][9] |
| Rice | < 0.5%[15] | 2–3%[15][9] |
| Sorghum | 2%[9] |
Notes: The newer highly-cultivated brewing barley varieties tend to have a lower β-glucan content compared to older varieties.[6] The β-glucans from oats are suggested to be more readily extractable than those in unmalted barley,[2] although results are conflicting.[12] Arabinoxylans in rye are more readily extractable than those in barley.[12] Six-rowed barely cultivars have significantly higher arabinoxylan contents than two-rowed cultivars.[9]
β-glucan degradation[edit]
Several types of endo-β-glucanase enzymes degrade of β-glucan to glucan dextrins and oligosaccharides (i.e. shorter chains).[5][7] This activity is the largely result of two isoenzymes of endo-β-(1,3;1,4)-glucanase, which degrade both types of linkages in the β-glucan chain (the enzymes cut the β-glucan chains in random spots).[5][4][7][11][17] Degradation of β-glucan can only occur after it has been solubilized (released from the cell walls).[7] The optimal pH range for endo-β-glucanase activity is 4.5–4.8, although the activity is fairly consistent over the usual mash pH range.[17] Optimal temperature is 113–119°F (45–48°C), and rapid enzyme denaturation (inactivation) occurs above 122°F (50°C).[1][5][7][10] Most of the enzymes are generated during malting, although a limited amount of enzyme activity is present even in unmalted barley.[7]
β-glucan degradation mainly occurs during malting,[1][17] where up to 90% of the grain β-glucan is degraded, but this is highly dependent on the barley variety (genetics), the growing conditions, and on the malting process itself.[4][7] For example, floor-malted barley generally has lower β-glucan content.[7] After malting, the dry weight of the malted barley contains only around 0.2–1.5% β-glucan, depending on the level of modification achieved.[4] Poorly-modified malt contains higher amounts of intact/large β-glucans as a result of less degradation.[5][11]
Endo-β-glucanases are not the only enzymes involved in the breakdown of β-glucans. Exo-β-glucanases are also present in barley are active during malting. They release glucose from the non-reducing ends of β-glucan, with optimal pH of 4.5 and temperature 104°F (40°C). The loss of exo-β-glucanase activity during kilning is more severe than that of endo-β-glucanases. Since the exo-β-glucanase removes only one glucose unit every time it attacks the barley β-glucan molecules, it has comparatively less impact on the degradation of β-glucan.[7]
The majority of the endo-β-glucanase activity survives kilning,[7] and therefore some β-glucan degradation can occur when mashing with a low temperature rest such as 113–119°F (45–48°C).[5][17] At these low temperatures, β-glucan is solubilized slowly, reaching a peak level after 30 minutes, and then declining due to continuous degradation by endo-β-glucanase.[5] As a result, wort contains low levels of β-glucan while mashing below 131°F (55°C) (until the temperature is increased).[5] It's important to note that the action of endo-β-glucanase is limited during mashing because it cannot access the β-glucans bound to proteins in the cell walls.[3][5] It's also noteworthy that the endo-β-glucanase enzymes have improved thermostability and greater activity in low-oxygen mashes because reduced (non-oxidized) glutathione and other antioxidants protect the methionine residue in the active site of the enzyme from oxidation.[7][18][19] Fine milling can also help increase the speed of β-glucan degradation due to accelerated diffusion and enzymatic activity during the lower temperature rest,[5] however fine milling will ultimately increase β-glucan levels in the wort.[10]
Arabinoxylan degradation[edit]
Arabinoxylans are not degraded as extensively as the β-glucans because the enzymes that degrade them are produced late in the germination process.[20][2][9][21] Only about 20–50% of the arabinoxylans are degraded during malting.[4][9] Many enzymes are involved in this process, such as endo-β-(1,4)-xylanases, β-(1,4)-xylosidases, acetyl xylanase, feruloyl esterase, α-L-arabinofuranosidases, and others.[4][2][14][1][9] Among them, endo-β-(1,4)-xylanase is the primary degradation enzyme, which can randomly cut the β-(1,4) linkages in xylan chain.[14][1] Endo-xylanases solubilize arabinoxylan (increasing arabinoxylan extraction), and also degrade the soluble arabinoxylan into smaller fragments.[2][9][21] The arabinoxylan-degrading enzymes are more thermostable than is β-glucanase, with the vast majority surviving through malt kilning.[9] Endo-β-(1,4)-xylanase is active in the similar range as suggested for β-glucanase, optimally around 113–119°F (45–48°C) or a bit higher, across a wide pH range, and it is active during mashing up to around 149°F (60°C).[9][4][1][22] Regardless, it is known that a relatively large portion of the extractable arabinoxylans found in malt tend to survive the brewing process and pass into the final beer with little or no modification.[2][8][9]
Unmalted wheat contains an endo-xylanase inhibitor.[9][23] When 40% unmalted wheat is used during mashing, it may cause a 12–58% reduction of the arabinoxylan level (compared to 100% barley malt, Congress mashing), but also an increase of the average size of the wort arabinoxylans.[9] Boiling of the wheat extract for 30 minutes destroys most of the inhibitor. Barley and other cereal grains also contain such an inhibitor, but studies are scarce.[24][25]
Enzyme additives[edit]
Exogenous microbial β-glucanase and xylanase enzyme products can be used to help avoid problems associated with excessive NSPs.[14][26][9][12] These commercial products usually contain β-glucanase activity, xylanase activity, and cellulase (cellulose-degrading) activity, and therefore degrade all types of NSPs in grain.[27] These enzymes can be added during malting, mashing, or fermentation. However, the addition of heat-stable products during mashing is preferred because of the enhanced action of the enzymes at higher temperatures, the increased extract yield, and the inactivation of the added enzymes by wort boiling.[7] It is also notable that the concentration of ferulic acid is increased.[27] Glucabuster has received favorable reviews by conventional (high-oxygen) home brewers, especially with regard to lautering speed and efficiency.[28][29] On the other hand, low oxygen brewers avoid problems related to NSPs without needing these enzymes, and thus their usage should probably be avoided because NSP degradation results in decreased body and lower health benefits (see below).
| Product | Vendor |
|---|---|
| CellarScience Glucabuster (concentrated ß-glucanase xylanase and cellulase) | MoreBeer |
Extraction during mashing[edit]
As mash temperature reaches 140°F (60°C) and higher, the wort β-glucan level dramatically increases.[5][7][10] This release is attributed to the action of so-called "β-glucan solubilase" enzyme.[3][11] This alleged "enzyme" is not well-understood, and its action may be the result of the combined effect of multiple enzymatic and non-enzymatic processes (i.e. directly resulting from starch gelatinization bursting the cell walls).[7][1][4][5][10][30] Regardless of the exact mechanism(s), β-glucan extraction begins slowly around 122°F (50°C), greatly increases around 140°F (60°C), peaks at 149°F (65°C), and remains active up to around 163°F (73°C).[5][3][6][10] Around the peak range, the large majority of β-glucans are dissolved within the first 10 minutes of mashing, and extraction may continue over the course of an hour or more.[7][10] Ultimately, ~70% of the malt β-glucan is dissolved in wort after mashing.[7] Once β-glucan extraction occurs (above 140°F/60°C), the level remains constant in the mash.[5][31] It is not degraded because the β-glucanase enzymes have been denatured, and neither does β-glucan precipitate during mashing. Therefore, significant β-glucan extraction into wort cannot be avoided since these temperatures are required for starch degradation.[3] Normal levels for β-glucan in wort are in the range of about 100–800 mg/L.[7][32]
As with β-glucans, arabinoxylans are partly extracted during mashing. The mechanism is not fully understood, although arabinoxylan extraction appears to largely be the result of simple dissolution in hot water rather than resulting from enzymatic activity or starch gelatinization.[2][3][9][21] There does appear to be some enzymatic release at temperatures below 149°F (65°C).[22] Ultimately around 10–20% of barley malt arabinoxylan is extracted into the wort.[2][9][12] Small amounts of arabinose and xylose sugars are also released, as well as ferulic acid.[2][9]
Fine grists always produce wort with higher β-glucan and arabinoxylan levels than do coarse grists.[7][10][9][12] Thus, for poorly-modified malt, coarse milling may help lower wort β-glucan and arabinoxylan levels, if desired. The β-glucan content of fine grists can be 1.5 to 3-fold compared to coarse grist.[5] It's also noteworthy that continuous, frequent, or more intensive stirring of the mash increase arabinoxylan extraction.[22]
Beyond mashing, there are some changes in NSP content. Around 5–10% of arabinoxylans precipitate with proteins and polyphenols during wort boiling.[9] With the chilling of wort after boiling, approximately 30% of the β-glucans precipitate, allowing them to be removed.[7] Lastly, a small portion of the wort NSP content sediments in the fermenter during fermentation (precipitating as a result of increased alcohol level).[9]
Content in beer[edit]
A 2011 study of fifteen commercial beers showed an average β-glucan content of 248 mg/L,[33] although there is a fairly broad range reported by the literature.[15][4] The β-glucan level increases greatly when unmalted barley is used, bringing the level as high as 1,152 mg/L (mainly large β-glucans) with 20% barley adjunct.[7]
One study of commercial beers showed that arabinoxylan content ranges from 514 to 4,211 mg/L.[15] In all cases, the arabinoxylan content was considerably higher than β-glucan. Other studies showed similar results.[4][2][34][12] Arabinoxylan molecules also tend to be much larger than β-glucans as a result of the lesser degradation during malting.[20] Beers with wheat generally contain especially high levels of arabinoxylan because of the high arabinoxylan content of wheat. Arabinoxylans may account for up to 10% of the total carbohydrates in beer (which is mainly dextrins).[15]
Effects on brewing and beer[edit]
The effects of β-glucans and arabinoxylans are similar and will be discussed together.
- Lower extract - Proteins, β-glucans, and arabinoxylans are the primary components of cell walls in the barley endosperm. When these components are not sufficiently degraded (i.e. modified during malting), they can block starch-degrading enzymes from accessing the starch, leading to lower extract obtained from the malt during mashing.[1][35][2][15][7][17][9]
- Inaccurate iodine test results - β-glucans can react with iodine, causing a false reading of an iodine test.[36]
- Increased wort viscosity - Extraction of large NSP molecules increases wort viscosity.[1][4][2][6] This leads to slower recirculation and lautering speeds.[7][10][14][15][17][9] Shear forces can cause NSPs (and proteins) to aggregate, which further increases the wort viscosity.[37][3][7][36][38] However, the relevance of shear force is questionable at the home brewing scale because shear forces are much lower compared to those in large commercial operations.[39] Furthermore, NSPs are not the main contributor to wort viscosity and also the effect of wort viscosity is relatively small at the high temperatures used during mashing (especially when a mash-out step is used).[37]
- Gel formation - NSPs are associated with the formation of gels during the brewing process.[10] In particular, arabinoxylans tend to form a gel under oxidative conditions.[3][15][6][9] Depending on the mashing process, these arabinoxylans can combine with proteins, lipids, and other carbohydrates to cause the formation of Oberteig, a dense doughy layer on top of the grain bed that may slow or stop wort flow during recirculation or lautering.[7][37] Low oxygen brewing can prevent the crosslinking of arabinoxylans with other macromolecules, and therefore prevent gel formation and subsequent wort flow issues (when combined with proper milling), even when using undermodified malts or adjuncts.
- Filtering problems - Unsurprisingly, high viscosity and/or gel formation can interfere with a filtration process (for brewers who filter their beer).[1][38][4][7][10][3][2][14][15][9]
- Haze - NSPs form particles that cause haze in beer.[1][10][2][14][15][17] This haze level increases with higher large NSP concentration, increased storage time, and higher ethanol concentration.[7][2] Furthermore, NSPs in beer may precipitate at low temperatures, causing "chill haze".[2][7]
- Full mouthfeel - NSPs enhance palate fullness, contributing to what is perceived as a more viscous, thick, and smooth mouthfeel.[40][13][34][12]
- Foam stability - Arabinoxylans have beneficial effects on beer foam stability.[13][21][9]
- Dietary benefits - Large (incompletely degraded) NSPs are a source of soluble fiber and therefore have potential benefits in the human diet.[20][30][41][8] Additionally, any NSP degradation products larger than glucose are not assimilable by the body and may constitute prebiotic material.[20]
- Antioxidant activity - Beer with higher levels of arabinoxylans has increased antioxidant activity due to the bound phenolic compounds, which can help improve flavor stability.[27]
Let's summarize the factors that mainly contribute to the various issues related to these molecules: Size matters. The size of the NSP molecules extracted into wort have a greater effect than the actual amount; smaller molecules (under 120kDa) typically do not cause significant negative effects.[1][7][9] Therefore, problems during brewing are often associated with the use of unmalted barley or the use of under-modified malt, both of which contain greater amounts of large NSPs that haven't been sufficiently degraded during malting.[7][2] Brewing problems are also more pronounced when using wheat malt because it has higher arabinoxylan content and therefore larger NSP molecules.[2] Fine milling causes greater NSP extraction. Lastly, oxygen ingress during mashing is a primary cause of wort flow issues because it encourages arabinoxylan gel formation. See Low oxygen brewing for an overview of best practices.
See also[edit]
To review:
- Li Y, Lu J, Gu G. Control of arabinoxylan solubilization and hydrolysis in mashing. Food Chem. 2005;90(1–2):101–108.
- Arabinoxylan, β-glucan and pectin in barley and malt endosperm cell walls: a microstructure study using CLSM and cryo-SEM
References[edit]
- ↑ a b c d e f g h i j k l m n o Evans E. Chapter 5: Mash substrates and enzymes: cell walls. In: Mashing. American Society of Brewing Chemists and Master Brewers Association of the Americas; 2021.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x Wang JM, Zhang GP. Chapter 5: β-glucans and arabinoxylans. In: Zhang G, Li C, eds. Genetics and Improvement of Barley Malt Quality. Springer; 2010:113–142.
- ↑ a b c d e f g h i j k l m n Kunze W, Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019.
- ↑ a b c d e f g h i j k l Burton RA, Collins HM, Fincher GB. Chapter 7: The role of endosperm cell walls in barley malting quality. In: Zhang G, Li C, eds. Genetics and Improvement of Barley Malt Quality. Springer; 2010:190–237.
- ↑ a b c d e f g h i j k l m n o p Kühbeck F, Dickel T, Krottenthaler M, et al. Effects of mashing parameters on mash β-glucan, FAN and soluble extract levels. J Inst Brew. 2005;111(3):316–327.
- ↑ a b c d e f g h i j Narziss L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Jin YL, Speers RA, Paulson AT, Stewart RJ. Barley β-glucans and their degradation during malting and brewing. Tech Q Master Brew Assoc Am. 2004;41(3):231–240.
- ↑ a b c Krahl M, Müller S, Zarnkow M, Back W, Becker T. Arabinoxylans and fructans in the malting and brewing process. Qual Assur Saf Crop Foods. 2009;1(4):246–255.
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag Egi A, Speers RA, Schwarz PB. Arabinoxylans and their behavior during malting and brewing. Tech Q Master Brew Assoc Am. 2004;41(3):248–267.
- ↑ a b c d e f g h i j k l Home S, Pietilä K, Sjoholm K. Control of glucanolysis in mashing. J Am Soc Brew Chem. 1993;51(3):108–113.
- ↑ a b c d Muller R. Factors influencing the stability of barley malt β-glucanase during mashing. J Am Soc Brew Chem. 1995;53(3):136–140.
- ↑ a b c d e f g h Langenaeken NA, De Schutter DP, Courtin CM. Arabinoxylan from non-malted cereals can act as mouthfeel contributor in beer. Carbohydr Polym. 2020;239:116257.
- ↑ a b c d Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b c d e f g h Yu J, Liu X, Guan L, Jiang Z, Yan Q, Yang S. High–level expression and enzymatic properties of a novel thermostable xylanase with high arabinoxylan degradation ability from Chaetomium sp. suitable for beer mashing. Int J Biol Macromol. 2021;168:223–232.
- ↑ a b c d e f g h i j k l m n Schwarz PB, Han JY. Arabinoxylan content of commercial beers. J Am Soc Brew Chem. 1995;53(4):157–159.
- ↑ Sapirstein HD. Bioactive compounds in wheat bran. In: Wrigley C, Corke H, Seetharaman K, Faubion J, eds. Encyclopedia of Food Grains. 2nd ed. Academic Press; 2016:268–276.
- ↑ a b c d e f g Kanauchi M, Bamforth CW. The relevance of different enzymes for the hydrolysis of β-glucans in malting and mashing. J Inst Brew. 2008;114(3);224–229.
- ↑ Bamforth CW, Muller RE, Walker MD. Oxygen and oxygen radicals in malting and brewing: a review. J Am Soc Brew Chem. 1993;51(3):79–88.
- ↑ Muller R. The formation of hydrogen peroxide during oxidation of thiol-containing proteins. J Inst Brew. 1997;103(5):307–310.
- ↑ a b c d Kanauchi M, Ishikura W, Bamforth CW. β-glucans and pentosans and their degradation products in commercial beers. J Inst Brew. 2011;117(1):120–124.
- ↑ a b c d Debyser W, Derdelinckx G, Delcour JA. Arabinoxylan and arabinoxylan hydrolysing activities in barley malts and worts derived from them. J Cereal Sci. 1997;26(1):67–74.
- ↑ a b c Vanbeneden N, Van Roey T, Willems F, Delvaux F, Delvaux FR. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008;111(1):83–91.
- ↑ Juge N, Payan F, Williamson G. XIP-I, a xylanase inhibitor protein from wheat: a novel protein function. Biochim Biophys Acta Proteins Proteom. 2004;1696(2):203–211.
- ↑ Goesaert H, Debyser W, Gebruers K, Proost P, Van Damme J, Delcour JA. Purification and partial characterization of an endoxylanase inhibitor from barley. Cereal Chem. 2001;78(4):453–457.
- ↑ Goesaert H, Gebruers K, Courtin CM, Delcour JA. Purification and characterization of a XIP-type endoxylanase inhibitor from Rice (Oryza sativa). J Enzyme Inhib Med Chem. 2005;20(1):95–101.
- ↑ Wang J, Bai Y, Shi P, et al. A novel xylanase, XynA4-2, from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry. World J Microbiol Biotechnol. 2011;27:207–213.
- ↑ a b c Szwajgier D, Pielecki J, Targoński Z. The release of ferulic acid and feruloylated oligosaccharides during wort and beer production. J Inst Brew. 2005;111(4):372–379.
- ↑ CellarScience® Glucabuster - Mashing Enzyme. MoreBeer website. Accessed May 27, 2022.
- ↑ Glucabuster and high efficiency. HomebrewTalk website. 2022. Accessed May 27, 2022.
- ↑ a b Bamforth CW. The Horace Brown Medal. Forever in focus: researches in malting and brewing sciences. J Inst Brew. 2020;126(1):4–13.
- ↑ Evans DE, Goldsmith M, Redd KS, Nischwitz R, Lentini A. Impact of mashing conditions on extract, its fermentability, and the levels of wort free amino nitrogen (FAN), β-glucan, and lipids. J Am Soc Brew Chem. 2012;70(1):39–49.
- ↑ Pahl R, Meyer B, Biurrun R. Wort and Wort Quality Parameters. In: Bamforth CW, ed. Brewing Materials and Processes: A Practical Approach to Beer Excellence. Academic Press; 2016.
- ↑ Szwajgier D. Dry and wet milling of malt: a preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J Inst Brew. 2011;117(4):569–577.
- ↑ a b Aerts G, Broekaert W, Courtin C, Delcour J, inventors; CBOK-ONDERZOEKSCENTRUM KAHO SINT-LIEVEN Katholieke Universiteit Leuven Cargill Inc, assignee. Arabinoxylo-oligosaccharides in beer. U.S. Patent No. US20100040731A1. February 14, 2007.
- ↑ Hu S, Dong J, Fan W, et al. The influence of proteolytic and cytolytic enzymes on starch degradation during mashing. J Inst Brew. 2014;120(4):379–384.
- ↑ a b Fix G. Principles of Brewing Science. 2nd ed. Brewers Publications; 1999.
- ↑ a b c Jin YL, Speers RA, Paulson AT, Stewart RJ. Effects of β-glucans, shearing, and environmental factors on wort filtration performance. J Am Soc Brew Chem. 2004;62(4):155–162.
- ↑ a b Sacher B, Becker T, Narziss L. Some reflections on mashing – Part 2. Brauwelt International. 2016;6:392–397.
- ↑ Pumping effects on wort and beer. American Homebrewers Association website forum. 2017. Accessed February 2022.
- ↑ Krebs G, Müller M, Becker T, Gastl M. Characterization of the macromolecular and sensory profile of non-alcoholic beers produced with various methods. Food Res Int. 2019;116:508–517.
- ↑ Saeed F, Pasha I, Anjum FM, Sultan MT. Arabinoxylans and arabinogalactans: A comprehensive treatise. Crit Rev Food Sci Nutr. 2011;51:467–476.
