Starch
Starch is made up of long chains of sugar, which are stored in granules within the kernels of cereal grains with the purpose of being used later as a source of energy for a newly growing plant. In beer production, this carbohydrate is instead used as the basis for a wonderful beverage. Starch is considered the most important component of malt, since it is the main ingredient of beer besides water. The malting and brewing processes are entirely designed around extracting and degrading the grain's starch. Brewing starts with the milling of the grain, which breaks apart the kernels so that the starch is accessable. Next, during mashing, hot water dissolves the starch (a process called gelatinization) and allows various enzymes to break apart the large starch molecules into sugars (a process called saccharification). This creates a sweet liquid called wort. The yeast can then ferment the sugars into alcohol. Armed with an understand of these processes, a brewer can tailor a mashing program to achieve the best results for starch degradation and extraction. If the starch degradation is incomplete for some reason, the cost of production increases, and intact starch molecules can cause a hazy or low-alcohol beer.[1]
Starch structure and organization[edit]
-
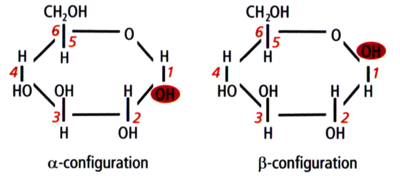
Glucose in alpha and beta configurations
-
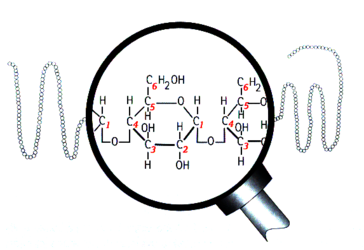
Structure of amylose
-
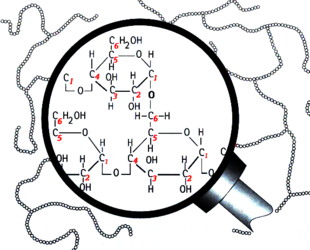
Branching in the structure of amylopectin
Structurally, starch is a polymer of glucose that can be classified into two categories: amylose and amylopectin. Amylose makes up 20–30% of the starch in barley and it consists of long unbranched or slightly-branched chains.[2][3][4] The rest is amylopectin, a much larger molecule consisting of branching chains.[5][6][7][8][9] The majority of glucose units are bound together with an α-1,4 glucosidic bond, which forms a straight chain. Branch points are formed by α-1,6 glucosidic bonds. This naming convention comes from the numbering of the carbon atoms in the glucose molecule. For example, 1,4 bonds are a connection between the 1st and 4th carbons of adjacent glucose units (see the above illustrations). The branch points are responsible for the unfermentable sugars (dextrins) in wort because the active enzymes in the mash generally cannot break down the starch molecules near a branch. Therefore, the degree of starch branching is a key variable that may impact beer quality, but unfortunately it is not measured by maltsters or brewers.[9]
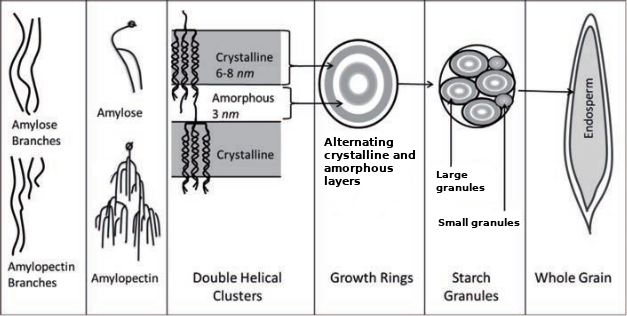
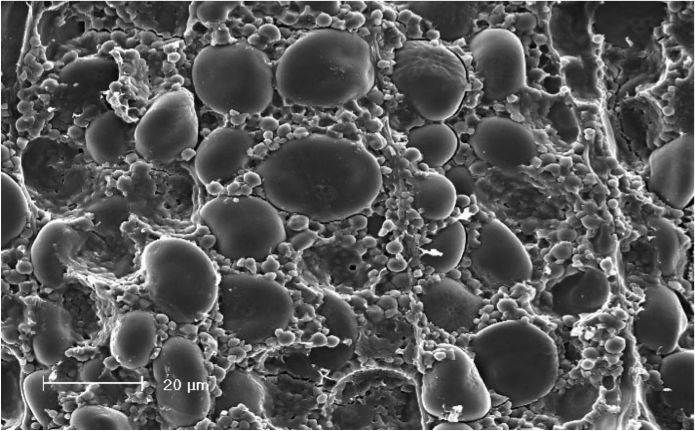
In cereal grain, the starch molecules form layers of crystalline and amorphous structure (like rings in a tree) in order to create granules, which make up the endosperm.[10] Starch granules in barley can be divided into two fractions: "large" granules (diameter ~20 µm) and "small" granules (diameter ~2 µm).[11][4] These types of barley starch granules contain different different amylose:amylopectin ratios, resulting in different structures and properties, especially gelatinization temperature (see below).[12][13] The "large" starch granules contain 70–95% of the starch mass.[14][15] The difference in gelatinization temperature is important because it impacts the amount of starch that is able to be extracted during mashing; the "small" granules have a significantly higher gelatinization temperature, which may require a step mash to extract or otherwise a substantial amount of starch is discarded with the spent grains.[16][3][17][18]
The ends of the starch chains have different structure due to the asymmetrical nature of the glucose molecule. The side with carbon #1 of the glucose unit is called the "reducing end" (RE) and carbon #4 is called the "non-reducing end" (NRE). Amylose has one RE and one NRE, while amylopectin has one RE and many NREs.[19] The ends differ in their chemistry and shape, which affects how the enzymes interact with the ends of the chains. (More on this below)
Gelatinization[edit]
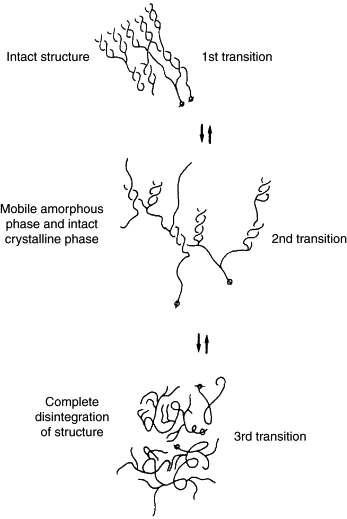
Gelatinization (also called "pasting"), which typically occurs during mashing, is the hydration of starch molecules. When exposed to hot water, starch granules swell and burst, losing their organized structure (think popcorn).[20][21][22][3] This physical process makes the starch much more accessible to enzymes.[23][24][4] If the starch is not fully gelatinized, it can potentially result in a lower yield, lower attenuation, filtration difficulties, possible scorching, and starch haze.[20][23] Therefore, the gelatinization temperature is important to consider when choosing a mashing schedule.[25] In the mash, gelatinization is followed by liquefaction and saccharification, an enzymatic process.
The gelatinization temperature of most cereals is between 149–176°F (65–80°C). However, it drops noticeably in the presence of starch-degrading enzymes (i.e. in malted grain).[20] For example, barley malt starch normally begins to gelatinize at 136–142°F (58–61°C), although it is variable.[20][26][27] It's important to understand that not all of the starch in a batch gelatinizes at the same temperature.[4] In particular, the "small" starch granules gelatinize at a significantly higher temperature than the majority of starch from the "large" granules, potentially as high as 186°F (80°C) in the case of barley malt.[21][24][28] Also be aware that different batches of grain will have slightly different gelatinization temperatures due to the effects of variable growth conditions and climatic influences.[25][29][30] Furthermore, the gelatinization temp can be influenced by milling—more intensive crushing lowers the gelatinization temperature.[24][31][32]
It can be problematic if too much starch in a batch of malt gelatinizes above 149°F (65°C).[4] β-amylase and limit dextrinase enzymes are quickly inactivated above this threshold (see below), which reduces the amount of starch degradation and therefore reduces the fermentability of the wort and/or decreases the yield.[33][34][26] Even with a proper step mash, this may result in a lower final attenuation as well as negative effects on flavor and mouthfeel.[25][35]
For so-called micronized, torrefied, and flaked grains, the starch is gelatinized as part of the production process and these grain products can be added directly to a normal malt mash in reasonable quantities (see Adjuncts).[36] With the exception of wheat, oats, and rye, raw unmalted cereals typically require a separate high-temperature gelatinization step to be used in a mash because the gelatinization temperature is too high to achieve during a normal mashing process (see Cereal mash).[22][4]
| Grain | Typical gelatinization temperature |
|---|---|
| Barley malt | 140–149°F (60–65°C)[21][22][23][29][37][38][4] |
| Wheat | 126–147°F (52–64°C)[37][4][39] |
| Oats | 126–147°F (52–64°C)[4][39] |
| Rye | 120–158°F (49–70°C)[4][39] |
| Maize | 143–176°F (62–80°C)[37][4][39] |
| Sorghum | 156–185°F (69–85°C)[37][4][39] |
| Rice | 142–180°F (61–82°C)[37][4][39] |
| Potato | 133–156°F (56–69°C)[37][39] |
Starch degradation (saccharification)[edit]
During the mash, gelatinization is followed by the degradation of starch molecules into smaller sugars, a process called saccharification or conversion. Efficient saccharification requires a team of enzymes working together. The most important enzymes for this process are α-amylase, β-amylase, and limit dextrinase, all of which are provided by barley malt.[35] The degree of starch degradation will directly determine the success of the fermentation and will contribute significantly to the flavor, color, and stability of the final beer.[40]
Enzyme activity[edit]
| Enzyme | Optimal | Inactivation | |
|---|---|---|---|
| Temp | pH | ||
| α-amylase | 162–167°F (72–75°C) |
5.6–5.8 | 172–176°F (78–80°C) |
| β-amylase | 140–149°F (60–65°C) |
5.4–5.6 | 149–158°F (65–70°C) |
| Limit dextrinase | 140–149°F (60–65°C) |
5.4 | 149–167°F (65–75°C) |
| α-glucosidase | 95–113°F (35–45°C) |
4.6–6.0 | 113–131°F (45–55°C) |
Alpha and beta amylase[edit]

Starch degradation begins with α-amylase cleaving some of the α-1,4 bonds internally in the starch molecules. The early effect of this action quickly decreases wort viscosity, a stage called liquefaction.[20][41] α-amylase continues breaking more and more α-1,4 bonds inside the starch chains, creating successively smaller sugars made up of many glucose molecules (called oligosaccharides).[7][20][21] Degradation is slower at the chain-ends and ceases near the α-1,6 branch points.[38] Although the activity of α-amylase is not entirely random, it is much less specific than the other enzymes.[42] α-amylase is stable over a relatively wide temperature range and therefore it is active during the entire mash (if a modern mashing process is followed).[43]

Degradation proceeds with β-amylase cleaving the second α-1,4 bond from the ends of starch chains, producing maltose molecules.[7][24] β-amylase also produces glucose and maltotriose in lesser amounts.[21] Unlike α-amylase, β-amylase is greatly susceptible to thermal inactivation. Therefore, the window for its activity is somewhat narrow, stretching no more than about 10°F (5°C) above the point where the starch begins to gelatinize. The ongoing actions of α-amylase and limit dextrinase provide a continuously increasing availability of chain ends that β-amylase can attack (specifically, the non-reducing ends).[20]
Degradation by the amylase enzymes stops two or three glucose molecules away from the branch points of amylopectin since neither α-amylase nor β-amylase can break the α-1,4 bonds this near to a branch point, nor can they degrade the α-1,6 bond (branch point) itself.[7][27][44] Therefore the combined action of the amylases produces a significant portion of small branched sugars, which are called "limit dextrins", in addition to the fermentable sugars.
Limit dextrinase[edit]

The third enzyme involved in starch degradation is a debranching enzyme called limit dextrinase. Most significantly, it can break α-1,6 bonds, although it can also break α-1,4 bonds. Cleaving the branch points creates small linear chains which enables the degradation of "limit dextrins" into fermentable sugars.[38] Limit dextrinase and β-amylase are the main enzymes responsible for creating an easily fermentable wort, particularly when their activity is optimized using temperature and pH adjustment.[45][3][46][47][27][33][48] However, even under optimal conditions, limit dextrinase only degrades a modest portion of the α-1,6 bonds, mainly due to the presence of an inhibitor protein that prevents much of the activity.[35][42][38][49][20] About 20–25% of the starch remains as branched dextrins that carry through to the finished beer.[27] Limit dextrinase is active in a temperature range similar to β-amylase, although it is less rapidly degraded since the inhibitor protein also provides thermal protection. In fact, the enzyme is only destroyed by boiling, and therefore limit dextrinase activity can be extensive during overnight mashing or in non-boiled beers, where it continues to increase fermentability.[50]
Alpha glucosidase[edit]
α-glucosidase (a class of enzymes that includes the so-called "maltase"[51]) degrades maltose, isomaltose, oligosaccharides, dextrins and starch at the ends of the chains, cleaving α-1,4 links preferentially and α-1,6 links more slowly, releasing glucose in each case.[38][7][47] This enzyme generally has insignificant activity during normal mashing due to thermal inactivation.[3][52][35][53][4] However, certain step mash schedules are uniquely designed to take advantage of the "maltase" activity (see Mashing).[29] In order for α-glucosidase to produce a significant amount of glucose, the wort must already have a high proportion of maltose (and maltose is only produced at a temperature range where α-glucosidase is inactivated).
Factors affecting saccharification[edit]
Biological processes are complex. Many different variables can affect the extent of saccharification in the mash and the resulting sugar profile.
Malt[edit]
The type of malt plays a huge role in the saccharification process. First, be aware that barley is an agricultural product with many different genetic varieties (i.e. breeds), and therefore some inconsistency should be expected when switching between different malts.[54][55][33][35][56] It is well-known that there are signicant differences in enzyme levels and their thermostability among different cultivars due to genetic factors.[4] Secondly, there are differences that can occur resulting from different growing conditions, which of course may vary considerably.[29] A notable example is the increasing starch gelatinization temperature resulting from dry growing conditions. Finally, the malting process has a large effect on the levels of enzymes in the malt. In particular, poorly-modified malt has much lower levels of enzymes.[57] Fortunately we have access to modern malts in which α-amylase, β-amylase, and limit dextrinase are usually present in adequate amounts to convert the starch during the mash—unless there is too high of a high percentage of unmalted grain (adjuncts) used.[3][27] However, variable enzyme levels can still affect the sugar profile. For example, dark malts (kilned to a greater extent) saccharify more slowly and provide lower final degrees of fermentation than light malts.[29] The survival of enzymes in malt is strongly dependent on the kilning conditions, with "lager" malts (kilned at lower temperature) generally containing significantly more enzymes.[38][3][58] Other factors during malting such as oxygen exposure can also affect enzyme levels and may be variable from lot to lot. FYI, 6-row barley contains "more enzymes" simply because it has smaller kernels and therefore less starch relative to the protein/enzyme content.[59]
Milling[edit]
Milling likely plays a role in saccharification by affecting the speed and extent of starch availability, enzyme availability, and even gelatinization temperature.[60] It's difficult to make general statements about these factors because the effects of home brew milling parameters have not been adequately studied. However, the saccharification process seems to be pretty forgiving of different milling specifications.[61][57]
Mashing parameters[edit]
There are three major mashing parameters and one minor parameter that influence enzyme activity in the mash and the resulting sugar composition of the wort.[20][29] These factors are further discussed in the Mashing and Brewing pH articles.
- Mash temperature(s)
- Mash pH value
- Mash duration
- Mash thickness
The effect of mash temperature is a complex balance between the starch gelatinization temperature, and temperature-driven rate of enzyme inactivation (or more generally, the optimal enzyme temperatures).[44] Higher mash temperatures increase the amount of starch that becomes gelatinized and therefore the amount that is extracted from the grain and degraded into sugars. Higher mash temperatures also increase the speed and effectiveness of the enzymes, but also increase the rate at which the enzymes become thermally inactivated. If the enzymes are inactivated too quickly, the wort may suffer from a lower degree of fermentability. A variety of factors influence the thermostability of the enzymes. However, with the exception of calcium level in the mash, these factors are generally outside the control of the brewer.[33]
Generally speaking, a mash pH around 5.4 (measured at room temperature) results in the most efficient starch degradation. Mash pH affects the activity of numerous enzymes beyond those involved with starch degradation.
Mash duration is an important factor that affects starch degradation since the enzymes require time to work. An shortened mash may result in a less fermentable wort and an extended mash may result in a more fermentable wort.[62] The maximum enzyme activity is reached after 10 to 20 minutes into the mash. After that it declines—rapidly at first, and then more gradually.[20]
In thick mashes, the amylases are more resistant than in thin ones due to the increased amount of protective colloids.[29] However, the effect of mash thickness is minimal and relatively unimportant for small-scale brewers.
FYI starch degradation can occur below gelatinization temperature.[63][13][64] In some cases, over 90% of the potential extract can be recovered by mashing two hours at 131°F (55°C), despite this temperature being well below the typical gelatinization temperature of barley starch.[38]
See also[edit]
External sources:
- Enzyme biochemistry - refer to The properties and genetics of barley malt starch degrading enzymes by Evans DE, Li C, and Eglinton JK 2009
References[edit]
- ↑ Ganbaatar C, Kubáň V, Kráčmar S, Valášek P, Fišera M, Hoza I. Liquid chromatographic determination of polyphenenols in Czech beers during brewing proces. Potravinárstvo. 2015;9(1):24–30.
- ↑ Buléon A, Colonna P, Planchot V, Ball S. Starch granules: structure and biosynthesis. Int J Biol Macromol. 1998;23(2):85–112.
- ↑ a b c d e f g Bamforth CW, Fox GP. Critical aspects of starch in brewing. BrewingScience. 2020;73(9/10):126–139.
- ↑ a b c d e f g h i j k l m n o Evans E. Mashing. American Society of Brewing Chemists and Master Brewers Association of the Americas; 2021.
- ↑ Kunze W. Raw materials. In: Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:39–107.
- ↑ Meussdoerffer F, Zarnkow M. Starchy raw materials. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ a b c d e Miedl-Appelbee M. Brewhouse technology. In: Stewart GG, Russell I, Anstruther A, eds. Handbook of Brewing. 3rd ed. CRC Press; 2017.
- ↑ Holbrook CJ. Brewhouse operations. In: Smart C, ed. The Craft Brewing Handbook. Woodhead Publishing; 2019.
- ↑ a b Fox GP. Starch in brewing applications. In: Sjöö M, Nilsson L, eds. Starch in Food. 2nd ed. Woodhead Publishing; 2017:633–659.
- ↑ Ratnayake WS, Jackson DS. Starch Gelatinization. In: Advances in Food and Nutrition Research. Academic Press; 2008;55:221–268.
- ↑ Chmelík J, Krumlová A, Budinská M, et al. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field-flow fractionation. J Inst Brew. 2001;107(1).
- ↑ Szwajgier D. Dry and wet milling of malt. A preliminary study comparing fermentable sugar, total protein, total phenolics and the ferulic acid content in non-hopped worts. J Inst Brew. 2011;117(4):569–577.
- ↑ a b Yu W, Zhai H, Xia G, et al. Starch fine molecular structures as a significant controller of the malting, mashing, and fermentation performance during beer production. Trends Food Sci Technol. 2020;105:296–307.
- ↑ Krottenthaler M, Back W, Zarnkow M. Wort production. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ Langenaeken NA, De Schepper CF, De Schutter DP, Courtin CM. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019;295:138–146.
- ↑ Keßler M, Kreisz S, Zarnkow M, Back, W. Do brewers need a starch modification index? Brauwelt International. 2008;1:52–55.
- ↑ Slack PT, Baxter ED, Wainwright T. Inhibition by hordein of starch degradation. J Inst Brew. 1979;85(2):112–114.
- ↑ Cozzolino D, Degner S. An overview on the role of lipids and fatty acids in barley grain and their products during beer brewing. Food Res Int. 2016;81:114–121.
- ↑ Lewis MJ, Young TW. Brewing. Springer; 2001:234.
- ↑ a b c d e f g h i j Kunze W. Wort production. In: Hendel O, ed. Technology Brewing & Malting. 6th ed. VLB Berlin; 2019:219–265.
- ↑ a b c d e Krottenthaler M, Back W, Zarnkow M. Wort production. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ a b c Fix G. Principles of Brewing Science. 2nd ed. Brewers Publications; 1999.
- ↑ a b c Slack PT, Wainwright T. Amylolysis of large starch granules from barleys in relation to their gelatinisation temperatures. J Inst Brew. 1980;86:74–77.
- ↑ a b c d Mousia Z, Balkin RC, Pandiella SS, Webb C. The effect of milling parameters on starch hydrolysis of milled malt in the brewing process. Process Biochem. 2004;39(12):2213–2219.
- ↑ a b c Sacher B, Becker T, Narziss L. Some reflections on mashing – Part 2. Brauwelt International. 2016;6:392-397.
- ↑ a b Fox GP, Staunton M, Agnew E, D'Arcy B. Effect of varying starch properties and mashing conditions on wort sugar profiles. J Inst Brew. 2019;125(4):412–421.
- ↑ a b c d e MacGregor AW, Bazin SL, Macri LJ, Babb JC. Modelling the contribution of alpha-amylase, beta-amylase and limit dextrinase to starch degradation during mashing. J Cereal Sci. 1999;29(2):161–169.
- ↑ Langenaeken NA, De Schepper CF, De Schutter DP, Courtin CM. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019;295:138–146.
- ↑ a b c d e f g Narziss L, Back W, Gastl M, Zarnkow M. Abriss der Bierbrauerei. 8th ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2017.
- ↑ Pahl R, Meyer B, Biurrun R. Wort and Wort Quality Parameters. In: Bamforth CW, ed. Brewing Materials and Processes: A Practical Approach to Beer Excellence. Academic Press; 2016.
- ↑ Morrison WR, Tester RF, Gidley MJ. Properties of damaged starch granules. II. Crystallinity, molecular order and gelatinisation of ball-milled starches. J Cereal Sci. 1994;19(3):209–217.
- ↑ Warpala IWS, Pandiella SS. Grist fractionation and starch modification during the milling of malt. Food and Bioproducts Processing. 2000;78(2):85–89.
- ↑ a b c d Evans DE, Fox GP. Comparison of diastatic power enzyme release and persistence during modified Institute of Brewing 65°C and Congress programmed mashes. J Am Soc Brew Chem. 2017;75(4):302–311.
- ↑ Evans DE, Li C, Eglinton JK. The properties and genetics of barley malt starch degrading enzymes. In: Zhang G, Li C, eds. Genetics and Improvement of Barley Malt Quality. Springer; 2010:143–189.
- ↑ a b c d e Evans DE, Collins H, Eglinton J, Wihelmson A. Assessing the impact of the level of diastatic power enzymes and their thermostability on the hydrolysis of starch during wort production to predict malt fermentability. J Am Soc Brew Chem. 2005;63(4):185–198.
- ↑ Howe S. Raw materials. In: Smart C, ed. The Craft Brewing Handbook. Woodhead Publishing; 2019.
- ↑ a b c d e f Meussdoerffer F, Zarnkow M. Starchy raw materials. In: Esslinger HM, ed. Handbook of Brewing: Processes, Technology, Markets. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2009.
- ↑ a b c d e f g Briggs DE, Boulton CA, Brookes PA, Stevens R. Brewing Science and Practice. Woodhead Publishing Limited and CRC Press LLC; 2004.
- ↑ a b c d e f g Mosher M, Trantham K. Brewing Science: A Multidisciplinary Approach. 2nd ed. Springer; 2021.
- ↑ Warpala IWS, Pandiella SS. Grist fractionation and starch modification during the milling of malt. Food and Bioproducts Processing. 2000;78(2):85–89.
- ↑ Fratianni A. Enzyme application in brewing. MBAA podcast. May 2020.
- ↑ a b Vriesekoop F, Rathband A, MacKinlay J, Bryce JH. The evolution of dextrins during the mashing and fermentation of all-malt whisky production. J Inst Brew. 2010;116(3):230–238.
- ↑ Muller R. The effects of mashing temperature and mash thickness on wort carbohydrate composition. J Inst Brew. 1991;97(2):85–92.
- ↑ a b Lewis MJ, Young TW. Brewing. Springer; 2001:233–249.
- ↑ Stenholm K, Home S. A new approach to limit dextrinase and its role in mashing. J Inst Brew. 1999;105(4):205–210.
- ↑ Arends AM, Fox GP, Henry RJ, Marschke RJ, Symons MH. Genetic and environmental variation in the diastatic power of Australian barley. J Cereal Sci. 1995;21(1):63–70.
- ↑ a b Guerra NP, Torrado-Agrasar A, López-Macías C, et al. Use of Amylolytic Enzymes in Brewing. In: Preedy VR, ed. Beer in Health and Disease Prevention. Academic Press; 2009:113–126.
- ↑ McCafferty CA, Jenkinson HR, Brosnan JM, Bryce JH. Limit dextrinase — Does its malt activity relate to its activity during brewing? J Inst Brew. 2004;110(4):284–296.
- ↑ Fox GP. Starch in brewing applications. In: Sjöö M, Nilsson L, eds. Starch in Food. 2nd ed. Woodhead Publishing; 2017:633–659.
- ↑ Can an overnight mash recover a too hot mash? HomebrewTalk website. 2022. Accessed April 26, 2022.
- ↑ Andriotis VME, Saalbach G, Waugh R, Field RA, Smith AM. The maltase involved in starch metabolism in barley endosperm is encoded by a single gene. PLoS ONE. 2016;11(3):1–13.
- ↑ Muslin EH, Karpelenia CB, Henson CA. The impact of thermostable α-glucosidase on the production of fermentable sugars during mashing. J Am Soc Brew Chem. 2003;61(3):142–145.
- ↑ Muslin EH, Clark SE, Henson CA. The effect of proline insertions on the thermostability of a barley α-glucosidase. Protein Eng Des Sel. 2002;15(1):29–33.
- ↑ Yin C, Zhang GP, Wang JM, Chen JX. Variation of beta-amylase activity in barley as affected by cultivar and environment and its relation to protein content and grain weight. J Cereal Sci. 2002;36(3):307–312.
- ↑ Hu S, Yu J, Dong J, et al. Relationship between levels of diastatic power enzymes and wort sugar production from different barley cultivars during the commercial mashing process of brewing. Starch - Stärke. 2013;65:1–9.
- ↑ Eglinton JK, Langridgeand P, Evans DE. Thermostability variation in alleles of barley beta-amylase. J Cereal Sci. 1998;28(3):301–309.
- ↑ a b Kühbeck F, Dickel T, Krottenthaler M, et al. Effects of mashing parameters on mash β-glucan, FAN and soluble extract levels. J Inst Brew. 2005;111(3):316–327.
- ↑ Kneen E, Spoer JM. The limit-dextrinase activity of barley malts. Proceedings from the Annual meeting of American Society of Brewing Chemists. 1948;6(1):20–27.
- ↑ Mahalingam R. Shotgun proteomics of the barley seed proteome. BMC Genomics. 2017;18(44).
- ↑ Meddings PJ, Potter OE. Physical and chemical processes in mashing. Effect of particle size. J Inst Brew. 1971;77(3):246–252.
- ↑ Lauro M, Forssell PM, Suortti MT, Hulleman SHD, Poutanen KS. Alpha-amylolysis of large barley starch granules. Cereal Chem. 1999;76(6):925–930.
- ↑ Sammartino M. Enzymes in Brewing. Tech Q Master Brew Assoc Am. 2015;52(3):156–164.
- ↑ Sun Z, Henson CA. A quantitative assessment of the importance of barley seed alpha-amylase, beta-amyalse, debranching enzyme and alpha-glucosidase in starch degradation. Arch Biochem Biophys. 1991;284(2):298–305.
- ↑ Bathgate GN. A review of malting and malt processing for whisky distillation. J Inst Brew. 2016;122(2):197–211.